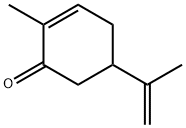
карвон
- английское имяCARVONE
- CAS №99-49-0
- CBNumberCB0773601
- ФормулаC10H14O
- мольный вес150.22
- EINECS202-759-5
- номер MDLMFCD00062996
- файл Mol99-49-0.mol
| Температура плавления | 230℃ |
| Температура кипения | 232℃ (760.0 Torr) |
| плотность | 0.963 g/cm3 (15℃) |
| FEMA | 2249 | CARVONE |
| показатель преломления | 1.710 |
| температура хранения | 2-8°C |
| растворимость | Chloroform (Slightly), Methanol (Slightly) |
| форма | Oil |
| цвет | Light Orange to Light Brown |
| Запах | at 100.00 %. minty licorice |
| Odor Type | minty |
| Номер JECFA | 380 |
| Диэлектрическая постоянная | 11.0(22℃) |
| Стабильность | Light Sensitive |
| LogP | 3.07 |
| Справочник по базе данных CAS | 99-49-0 |
| Вещества, добавляемые в пищу (ранее EAFUS) | CARVONE |
| Рейтинг продуктов питания EWG | 1-2 |
| FDA UNII | 75GK9XIA8I |
| Система регистрации веществ EPA | 2-Cyclohexen-1-one, 2-methyl-5-(1-methylethenyl)- (99-49-0) |
| Банк данных об опасных веществах | 99-49-0(Hazardous Substances Data) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P272:Не уносить загрязненную спецодежду с места работы.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P333+P313:При возникновении раздражения или покраснения кожи обратиться за медицинской помощью.
P363:Перед повторным использованием выстирать загрязненную одежду.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
карвон химические свойства, назначение, производство
Химические свойства
Carvone occurs as (S)-(+)- carvone ([α]18 D +64.3°,), (R)-(?)-carvone ([α]20 D ?62.5°), or racemic carvone. The optical isomers differ considerably in their sensory properties. They occur in high percentages in a number of essential oils. (+)-Carvone is the main component of caraway oil (about 60%) and dill oil; (?)- carvone occurs in spearmint oil at a concentration of 70–80%.Both (+)- and (?)-carvone are used to flavor a number of foods and beverages. (?)-Carvone is produced in much larger quantities and is mainly used in oral hygiene products.
Физические свойства
The carvones are colorless to slightly yellow liquids.(+)-Carvone has a herbaceous odor reminiscent of caraway and dill seeds, whereas (?)-carvone has a herbaceous odor reminiscent of spearmint. Depending on the reaction conditions, hydrogenation of carvone yields either carveol or dihydrocarvone, which are also used as flavor compounds. When treated with strong acids, carvone isomerizes to carvacrol.Вхождение
The optically active and inactive forms have been reported among the constituents of about 70 essential oils. The dextro form is present in carvi, Antheum graveolens, Antheum sowa, Lippia carviodora, Mentha arvensis, etc. The levo form is present in Metha vifidis var. crispa, Mentha longifolia from South Africa, Eucalyptus globules and several mint species. The racemic form is present in ginger grass, Litsea gutalemaleusis, lavender and Artemisia ferganensis. Reported found in citrus oil and juice (lemon, lime, orange), celery seed, anise, clove, coriander seed, calamus, caraway herb and dill seed.Использование
Carvone is useful for the treatment of various metabolic disorders and GI related disorders.Подготовка
In the past, (+)- and (?)-carvones were isolated by fractional distillation of caraway oil and spearmint oil, respectively. However, these carvones are now prepared synthetically, the preferred starting materials being (+)- and (?)- limonenes, which are converted into the corresponding optically active carvones. Since optical rotation is reversed in the process, (+)-limonene is the startingmaterial for (?)-carvone.Thepreferred industrialmethod of carvone synthesis utilizes the selective addition of nitrosyl chloride to the endocyclic double bond of limonene. If a lower aliphatic alcohol is used as solvent, limonene nitrosochloride is obtained in high yield. It is converted into carvone oxime by elimination of hydrogen chloride in the presence of a weak base. Acid hydrolysis in the presence of a hydroxylamine acceptor, such as acetone, yields carvone.
An alternative process for the production of (?)-carvone has recently been commercialized. Starting from (+)-limonene 1,2-epoxide, a regioselective rearrangement of the epoxide leads to (?)-carveol (trans- :[2102-58-1]; cis- :[2102-59-2]). Thereaction is effected by the use of a catalyst consisting of a combination of metal salts and phenolic compounds.
(?)-Carveol is subsequently oxidized to (?)-carvone by anOppenauer oxidation or by dehydrogenation in the presence of special catalysts.The reaction may also be performed as a one-pot reaction.
Определение
A ketone derived from the terpene dipentene. It is optically active, occurring naturally in both d- and l-forms.карвон запасные части и сырье
карвон поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +8617732866630 | China | 18147 | 58 | |
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 | |
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +86 18953170293 | China | 2930 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +86-29-87569266 15319487004 |
China | 3939 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 |