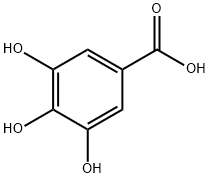
Галловая кислота
- английское имяGallic acid
- CAS №149-91-7
- CBNumberCB0158671
- ФормулаC7H6O5
- мольный вес170.12
- EINECS205-749-9
- номер MDLMFCD00002510
- файл Mol149-91-7.mol
| Температура плавления | 251 °C (dec.) (lit.) |
| Температура кипения | 259.73°C (rough estimate) |
| плотность | 1.694 |
| давление пара | 0Pa at 25℃ |
| показатель преломления | 1.5690 (estimate) |
| температура хранения | 2-8°C |
| растворимость | Methanol (Slightly), Water (Slightly, Heated) |
| пка | 4.41(at 25℃) |
| форма | Powder |
| цвет | Off-white |
| РН | 3.75(1 mM solution);3.22(10 mM solution);2.71(100 mM solution) |
| Биологические источники | plant fruit |
| Растворимость в воде | 12 g/L cold water |
| Мерк | 14,4345 |
| БРН | 2050274 |
| Стабильность | Stability Stable, but may discolour upon exposure to light. Hygroscopic. Incompatible with strong oxidizing agents, strong bases, acid chlorides, acid anhydrides. |
| ИнЧИКей | LNTHITQWFMADLM-UHFFFAOYSA-N |
| LogP | 0.7 |
| Справочник по базе данных CAS | 149-91-7(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 632XD903SP |
| Справочник по химии NIST | Benzoic acid, 3,4,5-trihydroxy-(149-91-7) |
| Система регистрации веществ EPA | Gallic acid (149-91-7) |
| UNSPSC Code | 41116107 |
| NACRES | NA.25 |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/37/38 | |||||||||
| Заявления о безопасности | 26-36-24/25-37/39 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | LW7525000 | |||||||||
| F | 8-9-23 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29182900 | |||||||||
| Банк данных об опасных веществах | 149-91-7(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 in rabbits (g/kg): 5.0 orally (Dollahite) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Галловая кислота химические свойства, назначение, производство
Описание
Gallic acid is a trihydroxybenzoic acid, a type of phenolic acid, a type of organic acid, also known as 3,4,5- trihydroxy benzoic acid, found in gallnuts, sumac, witch hazel, tea leaves, oak bark, and other plants. The chemical formula is C6H2(OH)3COOH. Gallic acid is found both free and as part of hydrolyzable tannins.Salts and esters of gallic acid are termed 'gallates'. Despite its name, it does not contain gallium.
Gallic acid is commonly used in the pharmaceutical industry. It is used as a standard for determining the phenol content of various analytes by the Folin - Ciocalteau assay; results are reported in gallic acid equivalents. Gallic acid can also be used as a starting material in the synthesis of the psychedelic alkaloid mescaline.
Gallic acid seems to have anti-fungal and antiviral properties. Gallic acid acts as an antioxidant and helps to protect human cells against oxidative damage. Gallic acid was found to show cytotoxicity against cancer cells, without harming healthy cells. Gallic acid is used as a remote astringent in cases of internal haemorrhage. Gallic acid is also used to treat albuminuria and diabetes. Some ointments to treat psoriasis and external haemorrhoids contain gallic acid.
Химические свойства
Gallic acid, also known as trihydroxybenzoic acid, is a colorless crystalline needles or prisms that is obtained from nutgall tannins. It is soluble in hot water, ether, ethanol, acetone and glycerin, insoluble in cold water, insoluble in benzene and chloroform and has a melting point of 235 to 240 °C (decomposition). When heated to 100-120°C, crystal water will be lost, and when heated above 200°C, carbon dioxide will be lost to generate pyrogallic acid (ie, pyrogallol). It is used in photography, tanning, ink manufacture and pharmaceuticals.Вхождение
Gallic acid is found in a number of land plants. It is also found in the aquatic plant Myriophyllum spicatum and shows an allelopathic effect on the growth of the blue-green alga Microcystis aeruginosa.In food
Areca nut
Bearberry (Arctostaphylos sp)
Bergenia sp
Blackberry
Hot chocolate
Juglans regia (Common walnut)
Mango in peels and leaves
Phyllanthus emblica (Indian gooseberry) in fruits
Raspberry
Syzygium aromaticum (clove)
Vinegar
wine
Witch hazel (Hamamelis virginiana)
White tea.
История
Gallic acid is an important component of iron gall ink, the standard European writing and drawing ink from the 12 th to 19th century with a history extending to the Roman empire and the Dead Sea Scrolls. Pliny the Elder (23-79 AD) describes his experiments with it and writes that it was used to produce dyes. Galls (also known as oak apples) from oak trees were crushed and mixed with water, producing tannic acid (a macromolecular complex containing gallic acid). It could then be mixed with green vitriol (ferrous sulfate) — obtained by allowing sulfate - saturated water from a spring or mine drainage to evaporate — and gum arabic from acacia trees; this combination of ingredients produced the ink.Gallic acid was one of the substances used by Angelo Mai (1782–1854), among other early investigators of palimpsests, to clear the top layer of text off and reveal hidden manuscripts underneath. Mai was the first to employ it, but did so "with a heavy hand", often rendering manuscripts too damaged for subsequent study by other researchers.
Early photographers, including Joseph Bancroft Reade (1801– 1870) and William Fox Talbot (1800 – 1877), used gallic acid for developing latent images in calotypes. It has also been used as a coating agent in zincography.
Использование
gallic acid is a potential bleaching agent and anti-oxidant, it is also astringent and potentially anti-microbial and anti-fungal. Scientists are finding that gallic acid may serve as a skin-lightening agent by inhibiting the action of the tyrosinase and peroxidase enzymes. Some studies indicate that it is more effective than hydroquinone when combined with the proper ingredients. It is also incorporated into anti-aging formulations for its ability to prevent mucopolysaccaride deterioration. It is a constituent of witch hazel and oak bark, among many other plants; however, it is generally obtained from nutgalls for commercial purposes.Методы производства
Gallic acid, a component of many tanning agents is an endogenous product in plants. The acid occurs free or bound to tannin [1401-55-4] (e.g., in divi-divi, oak bark, gallnuts, pomegranate roots, sumac, and tea). The acid is produced from tannin-rich aqueous gallnut extracts by acidic or alkaline hydrolysis. It is also obtained by using the enzyme tannase [9025-71- 2] or molds (Penicillium glaucum, Aspergillus niger) to cleave tannin by fermentation. Both the metabolism of gallic acid and its impact on plant growth enzymes have been studied.Определение
ChEBI: Gallic acid is a trihydroxybenzoic acid in which the hydroxy groups are at positions 3, 4, and 5. It has a role as an astringent, a cyclooxygenase 2 inhibitor, a plant metabolite, an antioxidant, an antineoplastic agent, a human xenobiotic metabolite, an EC 1.13.11.33 (arachidonate 15-lipoxygenase) inhibitor, an apoptosis inducer and a geroprotector. It is a conjugate acid of a gallate.Общее описание
Gallic acid is a phenol that has been found in C. sinensis and has diverse biological activities. It scavenges DPPH and hydroxyl radicals in cell-free assays (IC50s = 9.4 and 191 μM, respectively). Gallic acid (1-100 μM) reverses abscisic acid-induced inhibition of hypocotyl growth in A. caudatus seedlings. In vivo, gallic acid (21.8 g/kg) inhibits morpholine- and sodium nitrite-induced adenocarcinoma formation in mice. It also inhibits passive cutaneous anaphylaxis in mice when administered at a dose of 50 mg/kg.Реакции воздуха и воды
Sparingly water solubleПрофиль реактивности
Phenols, such as Gallic acid, do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases. Such heating may initiate polymerization of the organic compound. Phenols are sulfonated very readily (for example, by concentrated sulfuric acid at room temperature). The reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid.Угроза здоровью
Inhalation of dust may irritate nose and throat. Contact with eyes or skin causes irritation.Пожароопасность
Flash point data for Gallic acid are not available. Gallic acid is probably combustible.Побочные эффекты
It is a weak carbonic anhydrase inhibitor.Метаболизм
BiosynthesisChemical structure of 3,5- didehydro shikimate Gallic acid is formed from 3-dehydro shikimate by the action of the enzyme shikimate dehydro genase to produce 3,5-didehydro shikimate. This latter compound tautomerizes to form the redox equivalent gallic acid, where the equilibrium lies essentially entirely toward gallic acid because of the coincidently occurring aromatization.
Degradation
Gallate dioxygenase is an enzyme found in Pseudomonas putida that catalyzes the reaction :
gallate + O2 → (1E)-4-oxobut-1-ene-1,2,4-tri carboxylate.
Gallate decarboxylase is another enzyme in the degradation of gallic acid.
Conjugation
Gallate 1-beta-glucosyltransferase is an enzyme that uses UDPglucose and gallate, whereas its two products are UDP and 1-galloylbeta- D-glucose.
Методы очистки
Crystallise gallic from water. The tri-O-acetyl derivative has m 172o (from MeOH), and the anilide has m 207o(from EtOH). [Beilstein 10 H 470, 10 IV 1993.]использованная литература
Plant polyphenols (vegetable tannins): gallic acid metabolism. DOI:10.1039/NP9941100041Biotechnology of Food and Feed Additives DOI:10.1007/978-3-662-43761-2
Purification of Laboratory Chemicals DOI:10.5860/choice.50-6768
Gallic acid: a versatile antioxidant with promising therapeutic and industrial applications DOI:10.1039/C5RA01911G
Antioxidant properties and total phenolics content of green and black tea under different brewing conditions DOI:10.1007/S002170050406
Comparative study of eight well‐known polyphenolic antioxidants DOI:10.1211/0022357021693
Antagonistic Action of Phenolic Compounds on Abscisic Acid-Induced Inhibition of Hypocotyl Growth DOI:10.1093/JXB/31.6.1651
Галловая кислота запасные части и сырье
сырьё
запасной предмет
- Метил 3,4,5-тригидроксибензоат
- ink
- 2,3,4-тригидроксибензальдегид
- Галловая кислота этиловый эфир
- 1,2,3-Триметоксибензол
- 3,4,5-Триэтоксибензойная кислота
- 2,3,4-Триметоксибензальдегид
- 3,4,5-триметоксибензойной кислота
- Procyanidin B1
- (4'-O-метил) метилгаллат
1of4
Галловая кислота поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0744-8561603 +8619974416182 |
China | 26 | 58 | ||
| +86-0551-65418684 +8618949823763 |
China | 25356 | 58 | ||
| +8618007136271 | China | 5999 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-0311-87836622 +86-17333973358 |
China | 8051 | 58 | ||
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +86-18353166132 +86-18353166132 |
China | 983 | 58 | ||
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 |