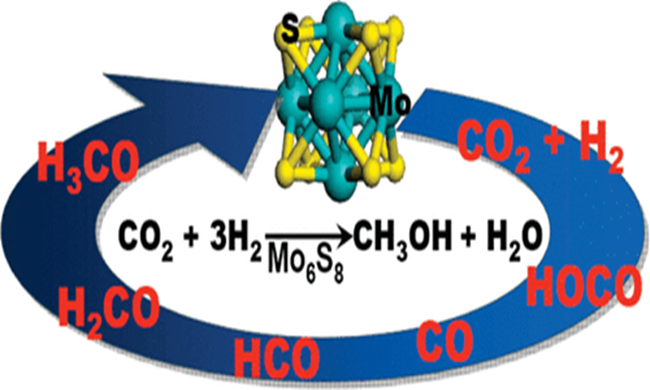
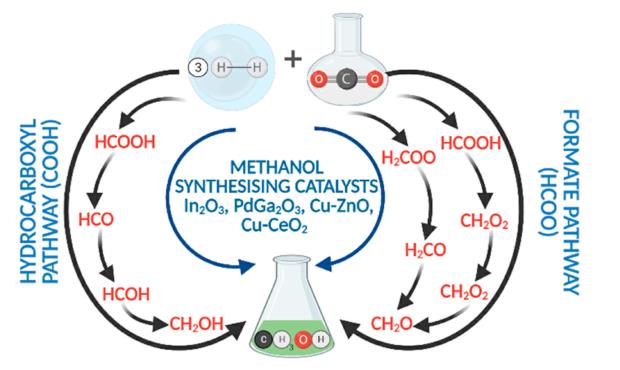
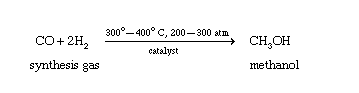
Why is methanol a polar molecule?

Occurrence
Small amounts of methanol are present in normal, healthy human individuals. One study found a mean of 4.5 ppm in the exhaled breath of test subjects.The mean endogenous methanol in humans of 0.45 g/d may be metabolized from pectin found in fruit; one kilogram of apple produces up to 1.4 g of pectin (0.6 g of methanol.)
Methanol is produced by anaerobic bacteria and phytoplankton.
Why is methanol a polar molecule?
Let’s talk about the methanol polarity.
To begin with, methanol, H3C−OH, is asymmetrical, so it could not be nonpolar (being 100% symmetrical in all directions means all dipole moments would cancel out completely).
The molecular geometry around oxygen in methanol is bent.
Oxygen is more electronegative than carbon or hydrogen, so the electron density is skewed towards oxygen.
Therefore, there is a net dipole with the negative end pointing through oxygen, and methanol is polar.
You may like
Related articles And Qustion
See also
Lastest Price from Methanol manufacturers

US $10.00/kg2025-04-21
- CAS:
- 67-56-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $0.00-0.00/kg2025-04-21
- CAS:
- 67-56-1
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons