METHANOL:Commercially Important Alcohols
Methanol (methyl alcohol) was originally produced by heating wood chips in the absence of air. Some of the carbohydrates in the wood are broken down to form methanol, and the methanol vapour is then condensed. This process led to the name wood alcohol as another common name for methanol. Methanol is synthesized commercially by a catalytic reaction of carbon monoxide (CO) with hydrogen gas (H2) under high temperature and pressure.
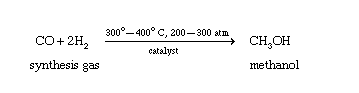
The mixture of carbon monoxide and hydrogen needed to make methanol can be generated by the partial burning of coal in the presence of water. By carefully regulating the amount of water added, the correct ratio of carbon monoxide to hydrogen can be obtained.
Methanol has excellent properties as a polar organic solvent and is widely used as an industrial solvent. It is more toxic than ethanol, however, and may cause blindness or death if large amounts are inhaled or ingested.

Methanol has a high octane rating and a low emission of pollutants—characteristics that make it a valuable fuel for automobile engines. From the late 1960s until 2006, the cars at the Indianapolis 500, the automobile race held annually at the Indianapolis Motor Speedway, were powered by methanol-burning engines. Methanol was once under consideration as a commercial motor fuel because it is cheaper than ethanol and can be made from natural gas and coal resources. However, increasing interest in ethanol-based fuels and difficulties involving the solvent properties of methanol, which cause problems with fuel systems—especially in fuel-injected cars—have resulted in diminished commercial interest in methanol fuels. Methanol tends to dissolve the plastic and rubber components employed in modern fuel systems, and different materials must be used that can survive exposure to methanol over long periods of time without dissolving or cracking.
Ethanol
Ethanol (ethyl alcohol) has been produced since prehistoric times, mostly through the fermentation of fruit juices. The fermented juice could be stored in a sealed container, and this primitive wine remained safe to drink throughout the winter. Many different sources can provide the sugars and starches that are broken down to simpler compounds during fermentation. Ethanol is called grain alcohol because it is often made from grains, such as corn (maize), wheat, rye, and barley. The grain is first boiled in water to produce the mash, which is incubated with malt (sprouted barley) to yield the wort. Malt provides an enzyme (diastase) that converts starches in the grain to the sugar maltose. The wort is incubated with brewer’s yeast, which secretes the enzyme maltase to convert maltose to glucose and the enzyme zymase to convert glucose to ethanol. Two of the six carbon atoms in glucose are oxidized to carbon dioxide (CO2); this oxidation provides energy to the yeast cells.
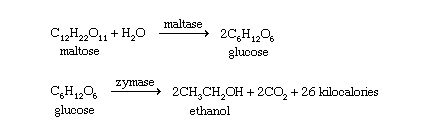
Fermentation yields a solution that is only about 12–15 percent alcohol because higher concentrations are toxic to the yeast cells. This solution can be distilled, however, to raise the ethanol content to as high as 95 percent.
Fermentation is a relatively expensive method of making ethanol. Industrial ethanol is more commonly synthesized by the high-temperature catalytic addition of water to ethylene (C2H4).
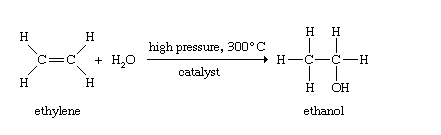
Ethanol is an excellent motor fuel with a high octane rating and low emissions; however, it should be used in a fuel system designed to withstand the alcohol’s tendency to dissolve plastic parts. Solutions of 10 percent ethanol in gasoline (gasohol) can be used in most cars without any adjustments. Today, ethanol fuels are typically made from natural products, such as corn or sugar.
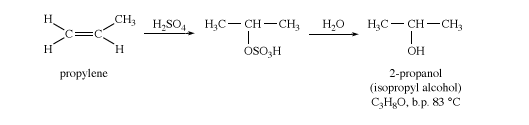
Isopropyl alcohol
Isopropyl alcohol (2-propanol) is made by indirect hydration of propylene (CH2CHCH3). Isopropyl alcohol is commonly used as an industrial solvent and as a rubbing alcohol applied to the skin. Although isopropyl alcohol is more toxic than ethanol, it has less of a drying effect on the skin, and it is not regulated and taxed by the U.S. government as is ethanol.
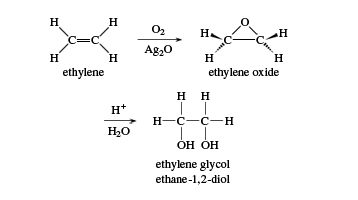
Ethylene glycol
The name ethylene glycol refers literally to “the glycol made from ethylene.” Its systematic name is ethane-1,2-diol. Ethylene glycol is commonly used as automotive antifreeze and as an ingredient in hydraulic fluids, printing inks, and paint solvents. It is also used as a reagent in making polyesters, explosives, alkyd resins, and synthetic waxes.
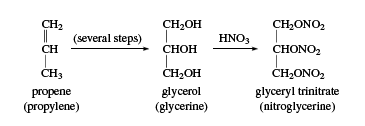
Glycerol
Glycerol (also called glycerine) is a sweet syrupy substance with three alcohol hydroxyl groups. Its systematic name is propane-1,2,3-triol. Glycerol was first obtained as a by-product of soap manufacture, through the saponification (hydrolysis in base) of fats. About 25 kg (60 pounds) of glycerol is obtained with each ton of soap. It can also be obtained by fermentation from molasses and sugar. During World War II, large quantities of glycerol were needed for the production of glyceryl trinitrate (nitroglycerin); this need was met by synthetic glycerol made from propylene, CH2=CH―CH3.
A large amount of glycerol is still used for making nitroglycerin, which is the primary explosive in dynamite and blasting gelatin. Nitroglycerin is also used as a coronary vasodilator (a drug that relaxes and expands blood vessels) for symptomatic relief of chest pain caused by poor circulation to the heart. Glycerol is also used as a solvent, moisturizing agent, plasticizer, antifreeze, and water-soluble lubricant. It is found in a wide variety of products, including foods, soaps, cosmetics, printing inks, hydraulic fluids, and pharmaceuticals.
Natural products
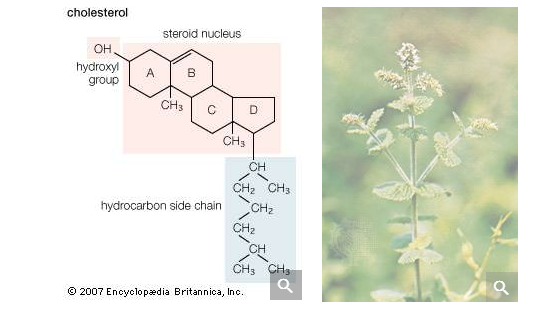
The common sources of methanol, ethanol, and isopropyl alcohol have been discussed above. Larger, more complicated alcohols are often isolated from volatile oils of plants by the process of steam distillation. The plant material is boiled in water, and the volatile oils are carried over by the steam, condensed, and separated from the water. Substances such as cholesterol, found in most animal tissues (and abundant in egg yolks), and retinol (vitamin A alcohol), extracted from fish liver oils, are examples of naturally occurring sources of alcohols.
Hydration of alkenes
The addition of water (hydration) across the double bond of an alkene yields an alcohol. Alkenes are available as products of coal tar and petroleum refining, and a variety of catalytic conditions can support the addition of water across the double bond. In most cases, water adds in the direction that places the new hydroxyl group on the more highly substituted end of the double bond according to the Markovnikov rule, as in acid-catalyzed hydrations. (The more highly substituted end of the double bond is the one that is bonded to more carbon atoms.)
Hydroboration-oxidation is also useful for adding water across the double bond of an alkene; however, hydroboration-oxidation gives an anti-Markovnikov orientation of the addition product, with the hydroxyl group adding to the less-substituted end of the double bond.
You may like
Related articles And Qustion
See also
Lastest Price from Methanol manufacturers

US $10.00/kg2025-04-21
- CAS:
- 67-56-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $0.00-0.00/kg2025-04-21
- CAS:
- 67-56-1
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons