Methanol Synthesis from H2 and CO2
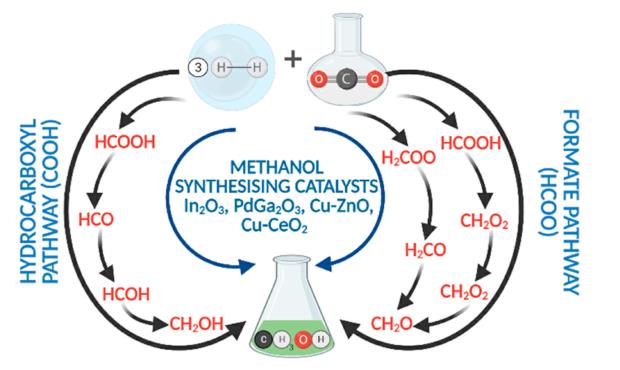
Catalytic CO2 hydrogenation to methanol has received considerable attention as an effective way to utilize CO2. In a research article from Ping Liu et. al., density functional theory was employed to investigate the methanol synthesis from CO2 and H2 on a Mo6S8 cluster.
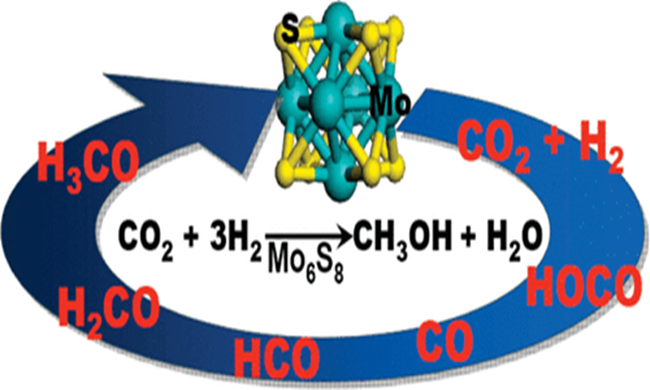
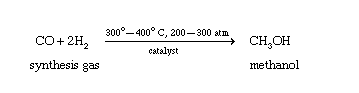
The Mo6S8 cluster is the structural building block of the Chevrel phase of molybdenum sulfide, and has a cagelike structure with an octahedral Mo6 metallic core. The calculations indicate that the preferred catalytic pathway for methanol synthesis on the Mo6S8 cluster is very different from that of bulklike MoS2. MoS2 promotes the C−O scission of HxCO intermediates, and therefore, only hydrocarbons are produced. The lower S/Mo ratio for the cluster compared to stoichiometric MoS2 might be expected to lead to higher activity because more low-coordinated Mo sites are available for reaction. However, the results show that the Mo6S8 cluster is not as reactive as bulk MoS2 because it is unable to break the C−O bond of HxCO intermediates and therefore cannot produce hydrocarbons. Yet, the Mo6S8 cluster is predicted to have moderate activity for converting CO2 and H2 to methanol. The overall reaction pathway involves the reverse water−gas shift reaction (CO2 + H2 → CO + H2O), followed by CO hydrogenation via HCO (CO + 2H2 → CH3OH) to form methanol. The rate-limiting step is CO hydrogenation to the HCO with a calculated barrier of +1 eV. This barrier is much lower than that calculated for a comparably sized Cu nanoparticle, which is the prototypical metal catalyst used for methanol synthesis from syngas (CO + H2). Both the Mo and S sites participate in the reaction with CO2, CO, and CHxO preferentially binding to the Mo sites, whereas S atoms facilitate H−H bond cleavage by forming relatively strong S−H bonds.
This study reveals that the unexpected activity of the Mo6S8 cluster is the result of the interplay between shifts in the Mo d-band and S p-band and its unique cagelike geometry.
References:
[1] PING LIU*. Methanol Synthesis from H2 and CO2 on a Mo6S8 Cluster: A Density Functional Study?[J]. The Journal of Physical Chemistry A, 2009, 114 11: 3743-4016. DOI:10.1021/jp906780a.You may like
Related articles And Qustion
See also
Lastest Price from Methanol manufacturers

US $10.00/kg2025-04-21
- CAS:
- 67-56-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $0.00-0.00/kg2025-04-21
- CAS:
- 67-56-1
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons