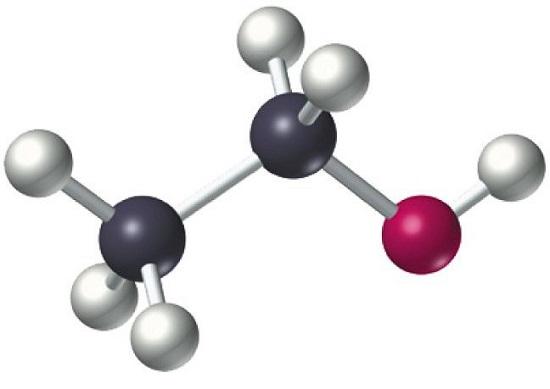
Overview of ethanol polarity
Ethanol is classified as a primary alcohol, meaning that the carbon that its hydroxyl group attaches to has at least two hydrogen atoms attached to it as well. Many ethanol reactions occur at its hydroxyl group.

Ethanol polarity
Let us start with the Lewis Structure and look and the polarity of the individual bonds in Ethanol based on the electronegativity difference between atoms.
Ethanol is a polar solvent. Its dipole moment is 1.69D. Now, a bond is generally considered polar if the electro negativity difference between the two bonded atoms is greater than or equal to 0.5. So, C-H bonds are not considered as polar.Hence the ethyl group is almost non-polar. But, it is the O-H bond that causes polarity to the ethanol molecule.
In O-H bond, O atom is in sp3 hybridized state and the geometry of orbitals around O atom is tetrahedral, with two vertices occupied by lone pairs of electrons. Now, O atom is bonded to ethyl group on one side and H atom on the other, with a bent shape. O-H and O-C bonds are both polar, oriented angularly with respect to each other. That is why ethanol molecule is polar.
You may like
Related articles And Qustion
See also
Lastest Price from Ethanol manufacturers

US $0.00/kg2025-11-28
- CAS:
- 64-17-5
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $10.00/kg2025-06-26
- CAS:
- 64-17-5
- Min. Order:
- 1kg
- Purity:
- 99.5%
- Supply Ability:
- 100 TON