Is Nitric oxide (NO) polar or non-polar?
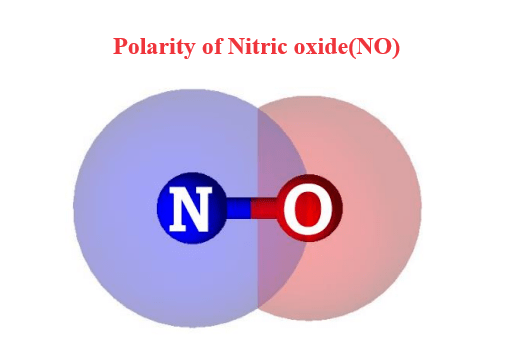
Polarity of Nitric oxide(NO)
Nitric oxide (NO) is a weakly polar molecule. Nitrogen and oxygen share two electron pairs and form a double covalent bond in a linear molecule. Oxygen (O) atoms are more electronegative than nitrogen (N) atoms. The oxygen atom is strongly attracted to the shared electron pair in the N=O bond. The difference in electronegativity results in a partially positive (δ) and partially negative (δ+-) charge on the N atoms and a charge on the O atoms. As a result, the N=O bond in the NO molecule is polar and exhibits a dipole moment value. Therefore, NO is a polar molecule with a dipole moment equal to 0.15 D.
Factors affecting NO polarity
Electronegativity
The greater the difference in electronegativity between bonded atoms in a molecule, the higher the bond polarity. Nitrogen is in group V-A (or 15) of the periodic table, so it has 5 valence electrons for bonding. Oxygen is in group VI-A (or 16) of the periodic table and has 6 valence electrons. Oxygen and nitrogen atoms share two electrons each to form a double covalent bond. Oxygen (E.N = 3.44) is more electronegative than nitrogen (E.N = 3.14). There is a 0.4 unit difference in electronegativity between the two atoms. Because of this electronegativity difference, the bonding electrons are close to the oxygen atom in the NO molecule.
The O-atom thus gains a partial negative (Oδ-) charge, while the nitrogen atom, less electronegative, obtains a partial positive (Nδ+) charge. In this way, oppositely charged poles develop in the NO molecule. However, the poles generated are weak as there is only a little electronegativity difference between the bonded atoms. Thus, NO is a weakly polar molecule.
Dipole Moment
The dipole moment is the product of electrical charge (Q) and bond length (r) between two bonded atoms. It is a vector quantity expressed in Debye (D) units. It is represented by a Greek symbol µ and measures the polarity of a bond. The dipole moment of any molecule depends on the difference in electronegativity between the bonded atoms. The greater the electronegativity difference, the higher the bond polarity, resulting in a high dipole moment value.
The dipole moment at the N=O bonding site ranges from Nδ+ to Oδ- due to the difference in electronegativity between N and O atoms. So, the dipole moment in NO molecule is weak. Therefore, the N=O bond in the NO molecule is polar with a dipole moment value of 0.15 D.
Molecular geometry
The nitric oxide (NO) molecule consists of a double N=O covalent bond. There are 11 valence electrons throughout the molecule. The oxygen atom has two pairs of lone electrons, while the nitrogen atom has one pair of lone electrons and one unpaired electron. The molecule has a linear shape, using to minimise repulsive effects due to the lone electrons on the N and O atoms. The bond angle is 180 degrees.