What is the therapeutic efficacy of the different dosage forms (injectable, oral and topical) of Diclofenac sodium?
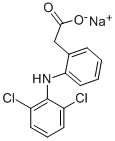
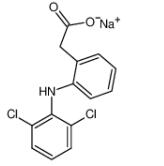
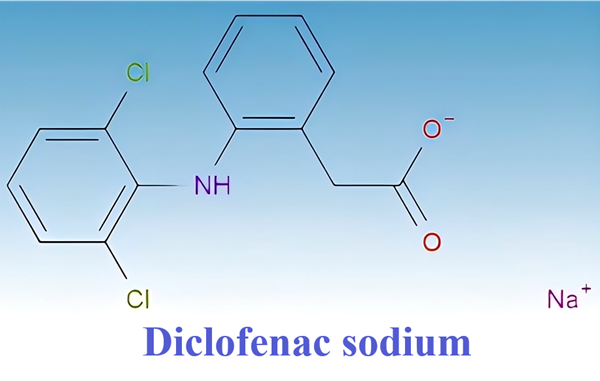
What is Diclofenac sodium?
Diclofenac sodium is a commonly used non-steroidal anti-inflammatory drug (NSAID) with antipyretic, anti-inflammatory and analgesic properties. It can be used to treat acute and chronic pain, including rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, muscle and ligament sprains, back pain, toothache, migraine and gout.
Injectable, Oral and Topical of Diclofenac sodium
It works by inhibiting cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2). However, diclofenac sodium inhibits COX-2 more selectively, which increases the risk of gastrointestinal and cardiovascular events. All NSAIDs, especially COX-2 inhibitors, are associated with an increased risk of myocardial infarction, heart failure, ischemic stroke, Kounis syndrome, and death.
Diclofenac sodium injection is characterised by a short onset of action and rapid onset of effect. Although anaphylaxis and anaphylactic shock secondary to diclofenac sodium injection are rare, anaphylactic shock due to intramuscular injection of diclofenac sodium has been reported. This report is also alerting healthcare professionals to this potentially life-threatening adverse reaction to diclofenac sodium and prompt treatment with epinephrine.
Topical formulations of nonsteroidal anti-inflammatory drugs (NSAIDs) are often considered safer alternatives to oral NSAIDs because of their lower systemic absorption.
In a randomised crossover study, the pharmacokinetics (PK), bioequivalence and safety of a 2% diclofenac sodium topical formulation twice daily (BID), a 1.5% diclofenac sodium topical formulation four times daily (QID) and oral diclofenac sodium were compared. The results showed comparable bioequivalence between the 2% and 1.5% topical solutions and lower systemic exposure (approximately 93% less) compared to the oral drug. Daily systemic exposure was comparable between the two formulations, with only a 12% difference in AUCss0-24 (p = 0.140). In addition, both topical solutions were eliminated later, with a 4- to 6-fold longer t1/2, compared with oral diclofenac. When comparing AUCss0-24 and Cmaxss across studies, the 2% solution was more dose stable than the 1.5% solution. Minor site-of-use reactions are the most common adverse effects of topical diclofenac.
References:
[1] DEBASHIS P SAHOO. Anaphylactic Shock After Intramuscular Diclofenac Sodium: A Case Report.[J]. Cureus, 2024, 16 2. DOI:10.7759/cureus.55279.[2] ROBERT J HOLT Jeffrey D K Tolu Taiwo. Bioequivalence of diclofenac sodium 2% and 1.5% topical solutions relative to oral diclofenac sodium in healthy volunteers.[J]. Postgraduate Medicine, 2015, 127 6. DOI:10.1080/00325481.2015.1058689.
You may like
Related articles And Qustion
Lastest Price from Diclofenac sodium manufacturers
US $0.00/kg2025-11-21
- CAS:
- 15307-79-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kgs

US $0.00/kg2025-05-07
- CAS:
- 15307-79-6
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg