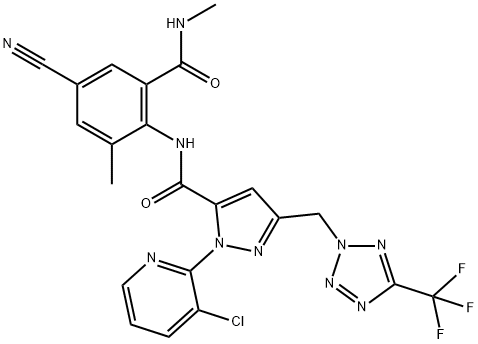
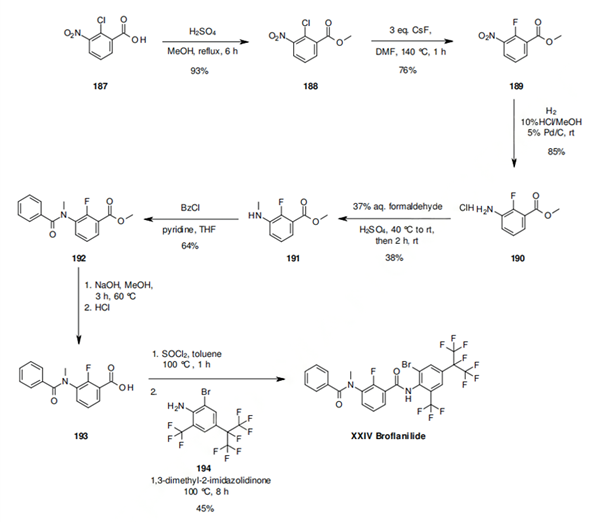
What is the synthesis of the insecticide Tetraniliprole?
Synthesis of Tetraniliprole
A plausible synthetic route to tetraniliprole involves the coupling of anthranilic amide 173 with the advanced intermediate pyrazole carboxylic acid 185.
1. Synthesis of intermediate 173
This involves bromination of 170 using hydrobromic acid and hydrogen peroxide, and further reaction of the resulting bromide 171 with copper cyanide to provide anthranilate 172. Reaction of 172 with methylamine in a basic methanol/sodium methanolate media affords anthranilamide 173 with an overall 69% yield.
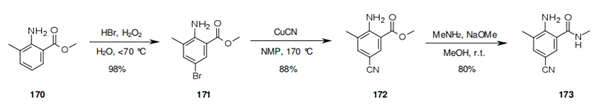
2. Preparation of tetraniliprole
Synthesis of the more complex intermediate 185 begins with electrophilic acylation of the vinyl ether 174 with trichloroacetyl chloride (175), to afford enone 176. Treatment of 176 with bromine,132 followed by displacement of the resulting bromide of 177 with potassium acetate yields 178, which upon reaction with hydrazine 179, leads to dihydropyrazole 180 which is then dehydrated to pyrazole 181 by treatment with oxalyl chloride. Hydrolysis of pyrazole 181 under acidic simultaneously hydrolyses both the CCl3 and acetate moieties to give the pyrazole carboxylic acid 182. Treatment of 182 with thionyl chloride followed by methanol quench leads to 183, which contains now the required methylene chloride side chain for nucleophilic substitution. This is executed by treatment of 183 with the tetrazole sodium salt 184 in the presence of potassium iodide to give the advanced intermediate 185 as the main component of a 94 to 6 regioisomeric mixture. In the final step of the synthesis of tetraniliprole (XXIII), the second amide bond is obtained by reacting acid 185 and aniline 173 in the presence of methanesulfonyl chloride and 2,6-lutidine.134 The latter step proceeds through an intermediate benzoxazinone as previously discussed for cyclaniliprole.
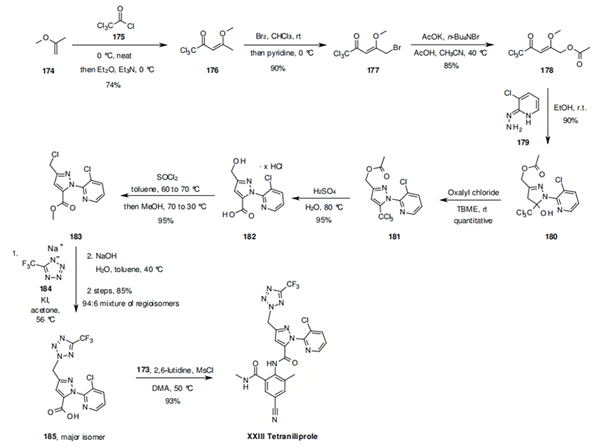
Introduction of Tetraniliprole
Tetraniliprole was announced by Bayer CropScience in 2014, as a new diamide insecticide. As with other anthranilic diamides, it acts as a ryanodine receptor modulator, but structurally differentiates itself from chlorantraniliprole (158) and cyantraniliprole (159) as the pyrazole moiety is substituted by a tetrazolylmethyl group rather than a bromide.