What is the synthesis of the insecticide Broflanilide?
Synthesis of Broflanilide
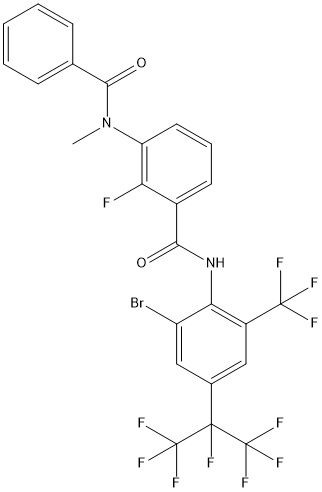
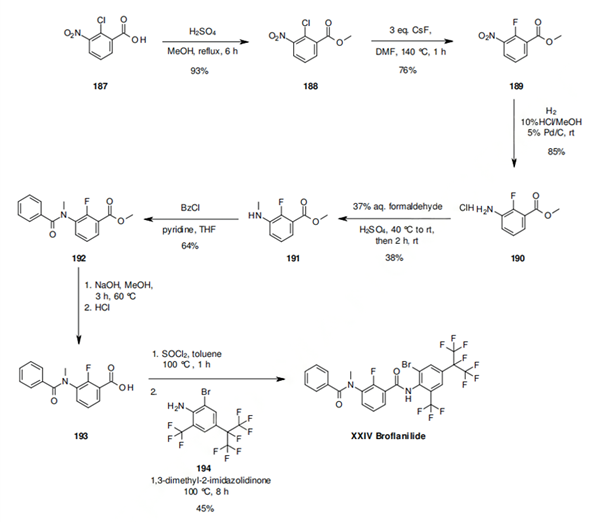
Scheme 1: The reported synthesis begins with protection of the acid function of 2-chloro-3-nitrobenzoic acid (187), as its methyl ester, followed by SNAr reaction of the chlorine in 188 with fluorine using cesium fluoride. This sequence potentially allows the introduction of a cheap source of fluoride like potassium fluoride. After reduction of the nitro group in 189, the amino group of molecule 190 is methylated through a variant of the Eschweiler-Clarke Reaction and subsequently condensed with benzoyl chloride to produce compound 192. Deprotection of the acid function, formation of the acid chloride followed by coupling with the aniline 194 provides broflanilide (XXIV).
Synthesis of aniline 194
The aniline required (194) is prepared starting from 2-trifluoromethyl aniline (195), radical condensation with heptafluoroisopropyl iodide (196), and subsequent bromination with N-bromosuccinimide (NBS).

Scheme 2: The last step in the synthetic route way is reported with low yield (45%). This is most likely
due to the fact that the aniline 194 is substituted with electron withdrawing groups and thus not
very reactive. An alternative approach, which adds the perfluorinated iso-propyl group unit at a
later stage and on a higher molecular weight intermediate is reported. Compound
193 is activated as its acid chloride 198 and then coupled 2-trifluoromethyl aniline (195) to yield
the advanced intermediate 199. Introduction of the perfluorinated iso-propyl group under the
radical conditions previously noted leads to 200, which is selectively brominated at the 2
position of the aromatic containing the perfluorinated iso-propyl group to give broflanilide
(XXIV).

Introduction of Broflanilide
Broflanilide is a new insecticide belonging to the meta-diamide class disclosed by Mitsui. Broflanilide exhibits larvicidal activity against Spodoptera litura. Broflanilide is, in fact, a procide where the methyl of the amide functionality is metabolized to liberate the desmethyl-broflanilide (186) (Figure 10). The molecule 186 is reported to be a non-competivite gamma-aminobutyric acid (GABA) receptor antagonist, but distinct from that of conventional non-competitive antagonists such as fipronil, picrotoxin, lindane, dieldrin, and α-endosulfan. It has been postulated that the binding mode of desmethyl-broflanilide is different from other compounds binding at the GABA receptor, and thus it is expected to be useful against pests resistant to cyclodienes and fipronil.

You may like
See also

US $0.00/kg2025-04-11
- CAS:
- 1207727-04-5
- Min. Order:
- 1kg
- Purity:
- 95
- Supply Ability:
- 20tons

US $0.00/kg2025-04-11
- CAS:
- 1207727-04-5
- Min. Order:
- 1kg
- Purity:
- 99
- Supply Ability:
- 20tons