What is the pH value of phosphoric acid?
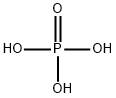
Phosphoric acid, also known as orthophosphoric acid or phosphoric(V) acid, is a mineral (inorganic) acid having the chemical formula H3PO4. The pH of a 0.15 M solution of phosphoric acid (H3PO4) is measured to be 1.54. Use this information to determine the value of the Ka for phosphoric acid.
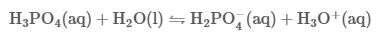
Just like other acids, the pH of a phosphoric acid solution is directly proportional to the concentration ofthe solute.It also varies depending on other factors such as temperature and the types of ions that
dissociate when the acidic substance is dissolved. For example, at 0.1 N aqueous solution, phosphoric acidhas a pH of 1.5. However, this only tells you the concentration of the hydronium ions at a logarithmic scale; it does notindicate the type of ions and their relative dissociations.
You may like
Related articles And Qustion
See also
Lastest Price from Phosphoric acid manufacturers

US $10.00/KG2025-04-21
- CAS:
- 7664-38-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $3465.00/KG2025-04-11
- CAS:
- 7664-38-2
- Min. Order:
- 1KG
- Purity:
- 85%
- Supply Ability:
- 400MT per month