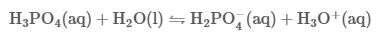Introduction of Phosphoric acid
General description
Diamine myricetin, also known as ampelopsin and AMP, is a diamine yellow diol xanthan compound with chemical structure of 3,5,7,3,4,5-hexazhe-2 and 3-diamine xanthan, as shown in The compound was first isolated by Kotake and Kubota from the leaves of Ampelopsis a. meliaefolia It is a polyphenol hydroxy flavonol with certain hydrophilicity and weak lipophilicity. Although it has good solubility in hot water, it has poor solubility in room temperature water; At the same time, dihydromyricetin is a polyphenol hydroxyl compound, which has poor stability and is easy to be oxidized and decomposed in the process of processing, storage and application, which affects its efficacy. (+)-dihydromyricetin is an optically active form of dihydromyricetin having (2R,3R)-configuration. It has a role as a metabolite, an antioxidant and an antineoplastic agent. It is a secondary alpha-hydroxy ketone and a dihydromyricetin. It is an enantiomer of a (-)-dihydromyricetin.[1]
Application and pharmacokinetics
Diamine myricetin has important application value in medicine, health care, food, animal husbandry, feed and other fields. DMY has a wide range of physiological activities, and its pharmacological effects mainly include; Scavenging free radicals, anti-oxidation,antiinflammatory and analgesic, reducing blood pressure, blood lipid, blood glucose, antithrombotic, anti-tumor, inhibiting pathogenic microorganisms, inhibiting hepatocyte deterioration, relieving alcohol protecting liver and protecting liver. It is reported that diamine myricetin has obtained invention patent authorization in the application field of preparing anti leukemia and pharyngeal cancer drugs, and is ready to enter the clinical trial stage as a class of new drugs; Diazo myricetin can be used as a natural preservative for the preservation of dairy products W and products susceptible to mold and bacteria. At the same concentration, it is better than the common preservative benzoic acid. It is an important food antioxidant and green additive; In addition, as an immune enhancer, oxymyricetin can promote the health and disease resistance of livestock and poultry, improve the growth and production performance of livestock and poultry, feed conversion rate, and improve poultry meat quality. It has a broad application prospect in animal husbandry. Therefore, it is of great practical significance to make full use of diamine myricetin. [2]
Dihydromyricetin in rattan tea has significant antibacterial, liver protecting, antioxidant and other effects, but the main pharmacodynamic material basis is not very clear, and the action mechanism is not very clear. Dihydromyricetin in China is rich in resources and has a wide range of sources, which has high development and utilization value. Previous studies have shown that dihydromyricetin has a variety of biological activities and has broad application prospects.A large number of studies have shown that acetylation of Dihydromyricetin products can enhance drug stability and increase drug fat solubility, do not change the basic structure of drugs, and can be reduced into technical drugs by enzymatic or non enzymatic action in vivo.Studies have shown that rattan tea has a wide range of pharmacological activities, such as anti-inflammatory, antioxidant, anti alcohol and liver protection, anti-tumor, antibacterial and antithrombotic. Flavonoids represented by dihydromyricetin are the pharmacodynamic material basis of Tengcha,
Synthesis
At present, dihydromyricetin is mainly extracted from rattan tea and other plants:
Weigh rattan tea, double the amount of water, boil, filter out the residue, double the amount of water, boil and discard the residue. The filtrate is placed, filtered out, precipitated, dissolved with ethanol, filtered, and concentrated by a rotary evaporator to obtain the crystallization of total flavonoids. Purification was performed by recrystallization using a two solvent method. Firstly, dissolve the crude extract with times the weight of absolute ethanol and decolorize with a small amount of activated carbon, then filter while hot and evaporate most of the ethanol, and then pour the residual solution into times the weight of cold water for cooling and crystallization. After secondary recrystallization, the content of dioxygen myricetin is increased from above.
Solvent extraction methods include hot water extraction and organic solvent extraction. Hot water extraction method refers to soaking extraction, and the extraction times are generally more. Hot water extraction method generally has the advantages of simple extraction process, low requirements for extraction equipment, good safety, less environmental pollution, less investment and easy to realize industrialized large-scale production.[1,3]
Figure 1 The Synthetic Route of Oac-Dihydromyricetin
Toxicological and Safety
The antifungal activity and potential antifungal mechanisms of dihydromyricetin (DMY) against Aspergillus flavus (A. flavus). First of all, through susceptibility test, the minimum inhibitory concentration (MIC) of DMY preventing [4]
Storage and Safty
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is required to be sealed and not in contact with air. It should be stored separately from oxidants, acids, and edible chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with leakage emergency treatment equipment and suitable storage materials.
References
1.Yang Wuxin: synthesis and pharmacological activity of Dihydromyricetin derivatives: Hunan University of traditional Chinese medicine, 2012.
2.Wang Chenguang: Study on the configuration and crystal form of dihydromyricetin in Tengcha based on pharmacokinetic properties: Huazhong University of science and technology, 2016.
3.Yuan Juan: extraction of Dihydromyricetin, preparation and physiological activity of metal ion complexes: Guangdong University of technology, 2013
4.Li Qian Mechanism of Dihydromyricetin inhibiting the growth of Aspergillus flavus
General description
Cypermethrin is an excellent pyrethroid with high efficiency, low toxicity and low residue, and it is suitable for killing agricultural pests. Cypermethrin is amber viscous liquid or solid, and industrial products are yellow or brown viscous liquid. It is soluble in organic solvents such as acetone, ethanol, hexane, benzene, toluene, xylene, chloroform, and slightly soluble in water. It also can be ecomposed in strong alkali.
Application
Cypermethrin is a high-efficiency, low-toxic insecticide with strong contact effect and broad insecticidal spectrum. It is suitable for controlling pests such as rice, cotton, corn, wheat, vegetables, fruit trees, tea trees and so on. It can also be used to control animal husbandry pests and sanitary pests. Its efficacy is second only to deltamethrin, and is about three times that of permethrin and cyanoether valerate. Approximately 2 g per mu per city can achieve the killing effect.
Synthesis
The synthesis of cypermethrin is divided into four steps. The first step is the preparation of permethrin acid. Heat and stir in a water bath containing stirring and refluxing dipermethrin acid chloride and ethanol. After the beginning of reflux, NaOH solution is added dropwise to carry out the saponification reaction. The temperature of the reaction liquid is maintained at 80-85°C, and the stirring time is about 4 hours. The pH value of the material is about 8-9, and the reaction is over. The ethanol is evaporated at atmospheric pressure, and the remaining pale yellow liquid is an aqueous solution of sodium permethrin. Add an appropriate amount of water and extract twice with 30 ml of benzene to remove the compounds that have not undergone saponification reaction. The separated aqueous phase is acidified with 1:1 hydrochloric acid (volume ratio) to a pH of about 3, and permethrin acid crystallizes out. After the crystallization is complete, suction filtration is performed, and the filter cake is washed with benzene and water once each. After drying, permethrin acid is obtained.
Figure 2 of synthesis of permethrin acid
The second step is the preparation of permethrin acid chloride. After the permethrin acid and toluene are completely dissolved, thionyl chloride is added, and the oil bath is heated to about 85°C for constant temperature reaction. The lye absorbs the acid gas of hydrogen chloride and nitrogen dioxide generated by the reaction, and distills at elevated temperature to distill off excess thionyl chloride and toluene to obtain a crude product of dipermethrin acid chloride. Under reduced pressure distillation, collect 5-6 mmHg distillate.The obtained slightly yellow transparent liquid is a relatively pure product.
Figure 3 of synthesis of permethrin acid chloride
The third step is the preparation of 3-phenoxybenzaldehyde. The toluene solution of 3-phenoxybenzylidene chloride is heated in an oil bath under stirring, and an appropriate amount of inorganic acid or organic acid is added dropwise to carry out the acidic hydrolysis reaction when there is reflux. Maintain the internal temperature at 110°C and reflux for about 5 hours. After finishing the reaction, standing still, after separating the water layer, the materials are neutralized and washed with dilute alkali, and separated into the oil phase to obtain the crude ether aldehyde.
The fourth step is the synthesis of cypermethrin. Pour 3-phenoxybenzaldehyde, dipermethrin acid chloride and an appropriate amount of solvent toluene into a three-necked flask, add sodium cyanide, sodium carbonate, and 3-phenoxybenzyltriethylammonium chloride dropwise under vigorous stirring The salt solution dissolved in water, after dripping, keep stirring and reacting at slightly higher than room temperature for 5-6 hours to complete the reaction. Transfer the material to a separatory funnel and separate the water phase. The aqueous phase was extracted once with a small amount of toluene. The toluene layer was combined with the organic phase, and washed with water until neutral. After removing the solvent toluene, cypermethrin is obtained. The yield was 96.2%
Figure 4 of synthesis of cypermethrin
Reference
1. Sha Hong, Yu Mingxing, Zhang Chaozhang. Synthesis of Cypermethrin[J]. Journal of Anhui University (Natural Science Edition), 2018(01): 46-50.
General description
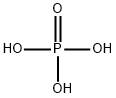
Phosphoric acid, also known as orthophosphoric acid (molecular structure formula H3PO4), is a colorless, transparent, viscous liquid or orthorhombic crystal, odorless and sour. 85% phosphoric acid is colorless, transparent or slightly light colored, thick liquid. Phosphoric acid is a phosphorus oxoacid that consists of one oxo and three hydroxy groups joined covalently to a central phosphorus atom. Phosphoric acid appears as a clear colorless liquid or transparent crystalline solid. The pure solid melts at 42.35°C and has a density of 1.834 g / cm3. Liquid is usually an 85% aqueous solution. Shipped as both a solid and liquid. Corrosive to metals and tissue. Used in making fertilizers and detergents and in food processing.the specific gravity is 1.70, the acid with high boiling point can be miscible with water in any ratio, and pyrophosphoric acid will be generated when the boiling point is 213 ℃ (loss of 1 / 2 water). When heated to 300 ℃, it becomes metaphosphoric acid. Relative density 181.834. Soluble in water, soluble in ethanol. Phosphoric acid is a common inorganic acid, which is a medium strong acid.[1]
Its acidity is weaker than strong acids such as sulfuric acid, hydrochloric acid and nitric acid, but stronger than weak acids such as acetic acid, boric acid and carbonic acid. When phosphoric acid reacts with sodium carbonate, different acid salts can be formed at different pH. It can stimulate skin, cause inflammation and destroy body tissue. Concentrated phosphoric acid has an erosive effect when heated in porcelain. Hygroscopic, sealed for storage. Commercially available phosphoric acid is a viscous concentrated solution containing H3PO4 82%. The high viscosity of phosphoric acid solution is due to the existence of hydrogen bonds in the solution.
Application
Phosphoric acid is an important chemical raw material, which is widely used in fertilizer industry, food industry, water treatment industry, electronic industry, coating industry and other fields. Food grade phosphoric acid is also a cheap sour chemical book agent. As a food additive, it is widely used in the production process of dairy products, meat products, aquatic products and various beverages. There is a great demand for food grade phosphoric acid at home and abroad, but the preparation methods have been used for a long time It has a role as a solvent, a human metabolite, an algal metabolite and a fertilizer. It is a conjugate acid of a dihydrogen phosphate and a phosphate ion.[2]
1.Phosphoric Acid is a colorless, odorless phosphorus-containing inorganic acid. Phosphoric acid is a sequestering agent which binds many divalent cations, including Fe++, Cu++, Ca++, and Mg++. Phosphoric acid is used in dentistry and orthodontics as an etching solution, to clean and roughen the surfaces of teeth where dental appliances or fillings will be placed. In addition, phosphoric acid is a constituent in bone and teeth, and plays a role in many metabolic processes. study demonstrate that the concentration of etching phosphoric acid had a significant effect on the microtensile bond strength of Adper Single Bond 2 tofluorotic teeth. [2]
2.The hydrothermal method was applied to food waste (FW) pretreatment with phosphoric acid as a catalyst. The content of soluble substances such as protein and carbohydrate in the FW increased after the hydrothermal pretreatment with phosphoric acid addition (65%). The SCOD approached approximately 29.0 g/L in 5% phosphoric acid group, which is almost 65% more than the original FW. The hydrothermal condition was 160℃ for 10 min, which means that at least 40% of energy and 60% of reaction time were saved to achieve the expected pretreatment effect. the percentage of propionic acid decreased and that of butyric acid increased.
3.With the vigorous development of China's new energy industry, the output of power battery as the core link is increasing. Lithium iron phosphate cathode lithium-ion battery has the advantages of long cycle life and low cost. It is widely used in new energy vehicles, electric bicycles, energy storage power stations and other fields.[3]
Synthesis and research
The production technologies of hot-process phosphoric acid thermal phosphoric acid is a kind of yellow phosphorus produced by electric furnace method, which is burned and oxidized into bimolecular phosphorus pentoxide by air (P4O10), Then P4O10 is further hydrated into phosphoric acid, and a large amount of heat is released during combustion and hydration .The total reaction formula of yellow phosphorus combustion :P4+5O2 =====P4O10At different temperatures, P2O5 has different hydration reactions, and different hydrated acids are transformed into orthophosphoric acid in the process.[4]
Figure1. Reaction heat recovery pipeline installed on graphite phosphorus combustion furnace
The process of thermal phosphoric acid mainly includes melting of yellow phosphorus, combustion of yellow phosphorus, hydration of phosphorus pentoxide gas, removal of arsenic, acid mist and capture of tail gas.[5]
Figure2. Steam boiler on graphite phosphorus burning furnace
Reference
1.Shen D., Wang K. & Yin J. et al., "Effect of phosphoric acid as a catalyst on the hydrothermal pretreatment and acidogenic fermentation of food waste," Waste Management, Vol.51(2016), pp.65-71.
2.Gu M., Lv L. & He X. et al., "Effect of phosphoric acid concentration used for etching on the microtensile bond strength to fluorotic teeth," Medicine, Vol.97, No.35(2018), p.e12093.
3.Yao Songsong, Wu Guoqing, Wang Hao, etc.: selective lithium extraction process of lithium iron phosphate battery powder, battery, No. 05, 2021, pp. 538-541.
4.Yang Yabin: development and trend of thermal phosphoric acid production technology, Yunnan chemical industry, No. 11, 2019, pp. 32-35
5.Gao Yongfeng: Discussion on innovative development ideas of China's phosphorus chemical industry, phosphate fertilizer and compound fertilizer, 2020, issue 02, pages 1-7
You may like
Related articles And Qustion
Lastest Price from Phosphoric acid manufacturers

US $10.00/KG2025-04-21
- CAS:
- 7664-38-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $3465.00/KG2025-04-11
- CAS:
- 7664-38-2
- Min. Order:
- 1KG
- Purity:
- 85%
- Supply Ability:
- 400MT per month