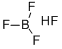How to synthesize Fluoroboric acid?
Introduction
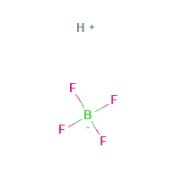
Fluoroboric acid is an inorganic, corrosive, strong acid with the chemical formula [H+][BF4-]. Available in various quantities, it is used as a precursor to fluoroborate salts and as a catalyst for alkylation and polymerization reactions. It is the base material for making fluoroborate and is widely used in industries such as oil, organic synthesis, light industry, and textile industry.

The ready availability of HBF4 and the ease of handling the adsorbed catalyst should make it cost-effective. Fluoroboric acid supported on silica gel efficiently catalyzes the acylation of structurally diverse phenols, alcohols, thiols, and amines under solvent-free conditions. Acid-sensitive alcohols are smoothly acylated without competitive side reactions[1].
Synthesis method
Pure fluoroboric acid is unknown. Aqueous solutions of 45-50% HBF4 are produced by combining technical grade[citation needed] boric acid and hydrofluoric acid in aqueous solution at room temperature. Three equivalents of HF react to give the intermediate boron trifluoride, and the fourth gives fluoroboric acid.
Aqueous solutions of fluoroboric acid can also be prepared by treating impure hexafluorosilicic acid with solid boric acid, followed by the removal of precipitated silicon dioxide. Anhydrous solutions can be prepared by treatment with acetic anhydride.
The detailed synthesis method is as follows:
1, anhydrous hydrogen fluoride is added to the water at normal temperatures and pressures; it is standby that dissolving generates 20% hydrofluoric acid;
2, in step 1, standby 20% hydrofluoric acid and boric acid is prepared burden by theoretical amount, under agitation 20% hydrofluoric acid is slowly joined in boric acid, controls temperature less than 45 ℃, and reaction is spent the night and namely got fluoroboric acid.
References
[1] Asit K. Chakraborti, Rajesh Gulhane. “Fluoroboric acid adsorbed on silica gel as a new and efficient catalyst for acylation of phenols, thiols, alcohols, and amines.” Tetrahedron Letters 44 17 (2003): Pages 3521-3525.
You may like
Related articles And Qustion
See also
Lastest Price from Fluoroboric acid manufacturers

US $0.00-0.00/KG2025-04-15
- CAS:
- 16872-11-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg

US $30.00-5.00/kg2025-03-07
- CAS:
- 16872-11-0
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10 tons