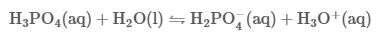Health hazard of phosphoric acid
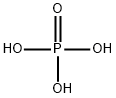
Pure phosphoric acid is normally in the form of a white crystalline solid.It has a melting point of 42.4°C.H3PO4 is non-toxic and non-volatile.Phosphoric acid falls into the category of weak acids. It is also referred to as orthophosphoric acid which helps us to easily distinguish it from other phosphoric acids such as polyphosphoric acid. Another name for this acid is phosphoric(V) acid. Phosphoric acid's formula is written as H3PO4.

Production
Phosphoric acid is produced industrially by one of two routes, wet processes and dry.
Wet process
In the wet process, a phosphate-containing mineral such as calcium hydroxyapatite and fluorapatite are treated with sulfuric acid.
Ca5(PO4)3OH + 5 H2SO4 → 3 H3PO4 + 5 CaSO4 + H2O
Ca5(PO4)3F + 5 H2SO4 → 3 H3PO4 + 5 CaSO4 + HF
Calcium sulfate (gypsum, CaSO4) is a by-product, which is removed as phosphogypsum. The hydrogen fluoride (HF) gas is streamed into a wet (water) scrubber producing hydrofluoric acid. In both cases the phosphoric acid solution usually contains 23–33% P2O5 (32–46% H3PO4). It may be concentrated to produce commercial- or merchant-grade phosphoric acid, which contains about 54–62% P2O5 (75–85% H3PO4). Further removal of water yields superphosphoric acid with a P2O5 concentration above 70% (corresponding to nearly 100% H3PO4). The phosphoric acid from both processes may be further purified by removing compounds of arsenic and other potentially toxic impurities.
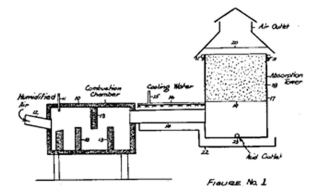
Dry process
To produce food-grade phosphoric acid, phosphate ore is first reduced with coke in an electric arc furnace, to give elemental phosphorus. Silica is also added, resulting in the production of calcium silicate slag. Elemental phosphorus is distilled out of the furnace and burned with air to produce high-purity phosphorus pentoxide, which is dissolved in water to make phosphoric acid.
Health hazard
Phosphoric acid, also known as orthophosphoric acid, is a triprotic acid that exists as a dense liquid. It is an irritant or corrosive to the skin, eyes, and other mucous membranes of both humans and laboratory animals. Its salts, though, exhibit a significantly lower irritancy potential.
Moderate toxicity has been observed in mice when exposed via the inhalation route. Phosphoric acid is not genotoxic nor carcinogenic, but phosphate salts have been reported to promote the activity of known carcinogens.
Exposures are treated typically by irrigation or flushing with water. Phosphoric acid has enjoyed significant interest as a food additive to various cola drinks, causing great controversy with regard to the potential for harmful effects.
You may like
Related articles And Qustion
Lastest Price from Phosphoric acid manufacturers

US $10.00/KG2025-04-21
- CAS:
- 7664-38-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $3465.00/KG2025-04-11
- CAS:
- 7664-38-2
- Min. Order:
- 1KG
- Purity:
- 85%
- Supply Ability:
- 400MT per month