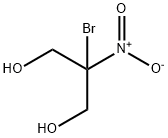
Toxicity of Bronopol
Bronopol strongly inhibits the growth of Gram positive and Gram negative Bacteria. It also is highly effective in eliminating the growth of Pseudomonas species, including pathogenic Pseudomonas aeruginosa. Its applications include cosmetic, pharmaceutical, toiletry and household products.

Uses
Bronopol is used as a microbiocide/microbiostat in oil field systems, air washer systems, air conditioning/humidifying systems, cooling water systems, papermills, absorbent clays, metal working fluids, printing inks, paints, adhesives and consumer/institutional products. A formulating technical material is also registered.
Toxicity
In laboratory animal studies measuring acute toxicity, technical grade bronopol has been shown to cause severe effects by the dermal route, placing it in Toxicity Category I (the highest of four categories) for dermal toxicity. It has been shown to produce irritation in eye and dermal irritation studies, placing it in Toxicity Category I for eye irritation and
Toxicity Category II for skin irritation. It is moderately toxic in oral toxicity studies, placing it in Toxicity Category II for oral toxicity. In an acute inhalation study, bronopol was found to be slightly toxic, placing it in Toxicity Category IV. This chemical is not a skin sensitizer based on a study using guinea pigs.
A 90-day oral toxicity study using rats indicated that bronopol is a severe gastrointestinal irritant.
A similar study in beagle dogs indicated only treatment related effects of increased liver and spleen weights in the high dose group. In a 90-day dermal toxicity study in rabbits, the NOEL for systemic toxicity was 2 mg/kg/day.
A chronic feeding/carcinogenicity study with rats resulted in high mortality, stomach lesions, and severe reduction in body weight gain. From a chronic dermal/carcinogenicity study, mice exhibited, moderate reduction in body weight gain. The Office of Pesticide Programs Reference Dose (RfD)/Peer Review Committee evaluated the carcinogenic potential of bronopol on April 18, 1995. The Committee classified bronopol as a Group E chemical ( one for which there is evidence of noncarcinogenicity for humans), based on a lack of evidence of cancer effects in acceptable studies with two animal species, the rat and mouse. Developmental toxicity studies were conducted using rats and rabbits. The results showed marginal to no effects in the rat study and effects only at the high dose level in the rabbit study.
A reproductive toxicity study using rats resulted in effects at the mid to high dose levels. The results included increases in kidney, thyroid and adrenal weights, decreases in liver weights, and decreased body weights. Bronopol was not mutagenic in four mutagenicity studies. Metabolism studies indicate that bronopol is primarily excreted in the urine.
You may like
Related articles And Qustion
See also
Lastest Price from Bronopol manufacturers

US $0.00-0.00/KG2025-04-21
- CAS:
- 52-51-7
- Min. Order:
- 1KG
- Purity:
- 99.66%
- Supply Ability:
- 20ton/month

US $6.00/kg2025-04-21
- CAS:
- 52-51-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month