What is the Lewis structure of Ethylene?
Introduction
Ethylene (ethene), CH2CH2, is the simplest molecule containing a carbon-carbon double bond. It is a gaseous plant hormone that plays pleotropic roles in plant growth, plant development, fruit ripening, stress responses, and pathogen defenses[1].
The Lewis structure of Ethylene
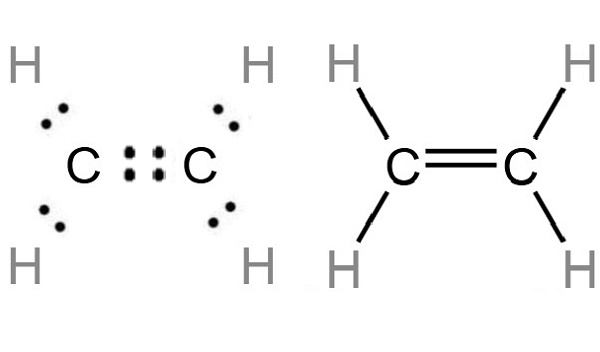
The Lewis structure, shown above, indicates that each C atom has zero pairs of electrons not used in bonding (called lone pairs) and four shared pairs of electrons (written between the atoms). The Lewis structure of ethylene indicates that there are one carbon-carbon double bond and four carbon-hydrogen single bonds. Experimentally, the four carbon-hydrogen bonds in the ethylene molecule are identical. Because each carbon is surrounded by three electron groups, VSEPR theory says the molecule should have a trigonal planar geometry.
Drawing the Lewis structure of ethylene requires the following five steps.
Step 1 Find the total number of electrons of the valance shells of carbon and hydrogen atoms
Carbon belongs to the group IVA elements series. Therefore, it has four electrons in its valence shell. Hydrogen is the first element in the periodic table that Hydrogen has only one electron in its valence shell. For a molecule, we add the number of valence electrons on each atom in the molecule, total valance electrons=4*2+1*4=12
Step 2 Total electron pairs
Total valance electron pairs = σ bonds + π bonds + lone pairs at valence shells
Total electron pairs are determined by dividing the number of total valence electrons by two. For an ethene molecule, the number of pairs of electrons is 6.
Step 3 Center atom selection
Hydrogen cannot be a center atom because its valence is limited to one, and Hydrogen can keep only two electrons in its valence shell. Therefore, both the carbon atoms should be placed in the center, and the remaining 4 hydrogen atoms will surround it. The following sketch can be proposed for ethene.
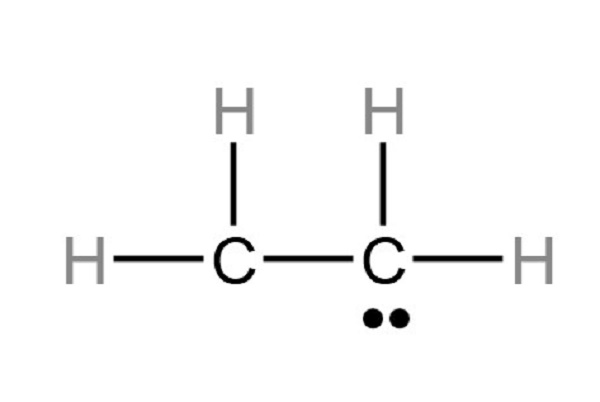
Step 4 Put lone pairs on atoms
Only 10 valence electrons of the C2H4 molecule are used in the above structure. Only one valence electron pair remains to draw (as mark lone pairs) the rest of the structure. After marking electron pairs on atoms, we should mark the charges of each atom.
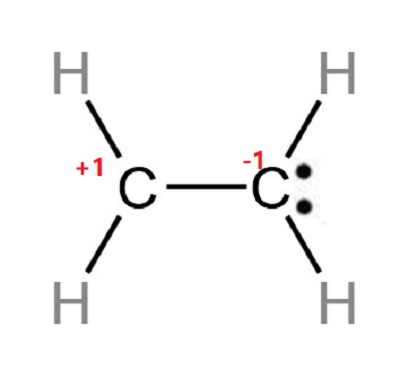
Step 5 Check the stability and minimize charges on atoms by converting lone pairs to bonds until the most stable structure is obtained.
When there are charges on many atoms in an ion or molecule, that structure is unstable. Therefore, we should reduce charges on atoms if possible. The Lewis structure of C2H4 could be shown below:
Formal charge = Valence Electrons – Unbonded Electrons – ½ Bonded Electrons
For hydrogen atom: Formal charge=1-0-2/2=0
For Carbon atom: Formal charge=4-0-8/2=0
Since the overall formal charge is zero, the above Lewis structure of C2H4 is most appropriate, reliable, and stable.
Reference
[1] Jing Li. "Enhanced CO2 Reduction to Ethylene with Hollow Cu2O Structure by Facet-controlled Etching." Nano (2023).