Ethylene: uses and Biosynthesis
Introduction
Ethylene (H2C=CH2) is the simplest of the organic compounds known as alkenes, which contain carbon-carbon double bonds. It is a colourless, flammable gas with a sweet taste and odour. Natural sources of ethylene include both natural gas and petroleum; it is also a naturally occurring hormone in plants, which inhibits growth and promotes leaf fall, and in fruits, it promotes ripening.
Ethylene is an essential industrial organic chemical. It is produced by heating either natural gas, especially its ethane and propane components, or petroleum to 800–900 °C (1,470–1,650 °F), giving a mixture of gases from which the ethylene is separated. The melting point of ethylene is −169.4 °C [−272.9 °F], and its boiling point is −103.9 °C [−155.0 °F].
Uses
About 80% of the ethylene used in western Europe, Japan and the USA produces polyethene (high density, low density and linear low density), ethylene oxide/ethylene glycols and ethylene dichloride/vinyl chloride. Significant amounts are also used to make ethylbenzene/styrene, oligomer products (e.g. alcohols and a-olefins), acetaldehyde/acetic acid and vinyl acetate.
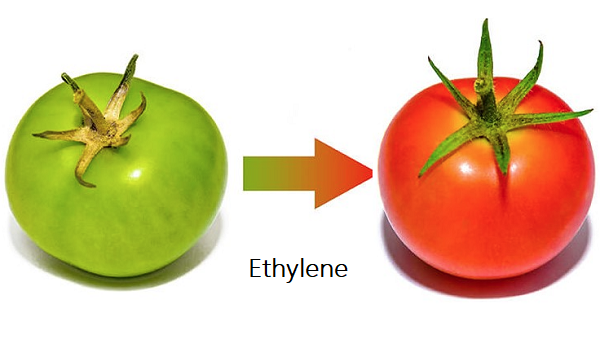
While most commercially produced ethylene is used as a feedstock for polymers and industrial chemicals, a relatively small amount is used for the controlled ripening of citrus fruits, tomatoes, bananas, and many other fruits, vegetables, and flowers. Endogenous production of ethylene in plant tissue generally increases rapidly during ripening. Application of ethylene to plants before the time of this natural increase not only initiates the ripening process but also increases endogenous ethylene production.
Biosynthesis
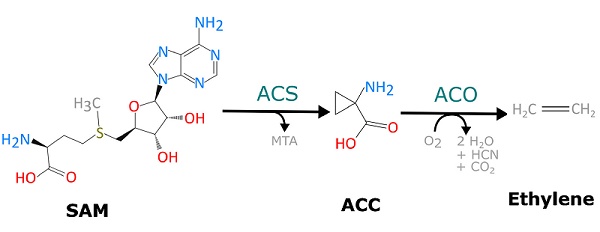
The ethylene biosynthesis pathway is relatively simple, occurring via only two committed enzymatic reactions. In the first step, the substrate S-adenosyl-l-methionine (SAM) is converted to ACC and 5′-methylthioadenosine (MTA) by the enzyme ACC synthase (ACS). In the second step, ACC is converted to ethylene, CO2 and cyanide by the ACC oxidase (ACO) enzyme. The toxicity of the cyanide by-product is rapidly dealt with by conversion to β-cyanoalanine by a group of β-cyanoalanine synthases. Because ACS and ACO are the only enzymes dedicated to ethylene biosynthesis, much of the regulation of overall ethylene production occurs by manipulating the transcription, translation and protein stability of these two enzymes. Additionally, metabolic regulation of ethylene production is achieved by ACC homeostasis, which encompasses ACC biosynthesis, transport and conjugation.