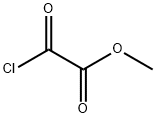
What is Methyl Oxalyl Chloride?
Feb 18,2020
Methyl oxalyl chloride is an important organic intermediate (building block) to synthetize substituted methyl oxalyl products. It can be used for regioselective synthesis of fused coumarins, cyclization to form substituted isoxazoles and heterocycles, intramolecular Wittig reactions, silyl enol ether acylation, and iron-mediated cleavage of C-C bonds.
The following example is about its application on the synthesis of methyl (2S)-1-(1,2-dioxo-2-methoxyethyl)-2-pyrrolidinecarboxylate [1].
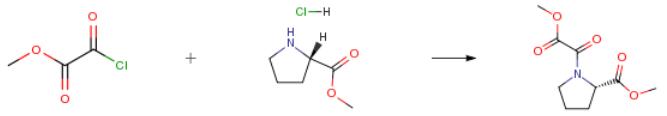
A solution of L-proline methyl ester hydrochloride (3.08 g; 18.60 mmol) in dry methylene chloride was cooled to 0° C. and treated with triethylamine (3.92 g; 38.74 mmol; 2.1 eq). After stirring the formed slurry under a nitrogen atmosphere for 15 min, a solution of methyl oxalyl chloride (3.20 g; 26.12 mmol) in methylene chloride (45 ml) was added dropwise. The resulting mixture was stirred at 0° C. for 1.5 hour.. After filtering to remove solids, the organic phase was washed with water, dried over MgSO4 and concentrated. The crude residue was purified on a silica gel column, eluding with 50percent ethyl acetate in hexane, to obtain 3.52 g (88percent) of the product as a reddish oil.
The following example is about its application on the synthesis of methyl 2-(2-tert-Butylphenylamino)-2-oxoacetate [2].
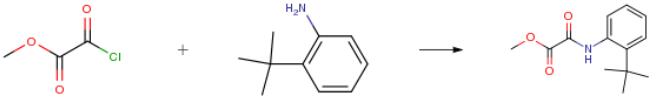
A 3-L, 3-necked round-bottomed flask, equipped with a mechanic stirrer and a thermal probe (under nitrogen) was charged with 2-tert-Butylaniline (109 g, 114 mL, 732 mmoles), triethylamine (81.4 g, 112 mL, 804 mmoles, 1.1 equiv.) and toluene (600 mL). The resulting mixture was stirred at a moderate speed at -30° C. An additon funnel was charged with Methyl chlorooxoacetate (100 g, 816 mmoles, 1.11 equiv.) with toluene (200 mL), and the mixture added to the reaction mixture at such a rate that the internal batch temperature is less than -20° C. After addition, the reaction was warmed to room temperature for an hour, quenched with water, then partitioned between E,tOAc/water. The aqueous phase was extracted with EtOAc (200 mL), and the combined organic layers were washed successively with KHSO4 (200 mL), NaHCO3 (sat'd) (200 mL) and brine (200 mL), then dried the organic over MgSO4.
The organic phase was concentrated by rotary evaporation, yielding the title compound as a pale yellow solid [160 g, 93.1percent]. The product thus obtained can be used directly for the subsequent reaction.
The following example is about its application on the synthesis of N-Cyclopropyl-N-(2-nitro-phenyl)-oxalamic acid methyl ester [3].
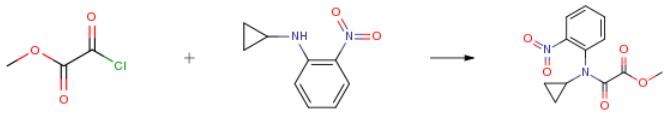
To a solution of cyclopropyl-(2-nitro-phenyl)-amine (32.0 g, 0.18 mol, 1.0 equiv) in dichloromethane (320 mL) was added triethylamine (18.2 g, 25.0 mL, 0.18 mol, 1.0 equiv and methyl oxalyl chloride (22.0 g, 16.5 mL, 0.18 mol, 1.0 equiv slowly at 0° C. After the addition was completed the reaction mixture was stirred at rt for 72 h. The reaction mixture was extraction from a sat. solution of NaHCO3 (300 mL) with dichloromethane (3*200 mL) and the combined organic phases dried over MgSO4. Purification by silica column chromatography using a MPLC system eluding with a mixture of heptane/ethyl acetate (2:1) afforded 45.2 g (95percent) of the final compound as a white solid.
References
1.GPI NIL Holdings, Inc. Nitrogen-containing linear and azepinyl/ compositions and uses for vision and memory disorders. US6335348[P], 2002, B1, Page column 18.
2.Karanewsky DS, Ternansky RJ, Linton SD, Dinh T. C-terminal modified oxamyl dipeptides as inhibitors of the ICE-ced-3 family of cysteine proteases. US2002/42376[P], 2002, A1.
Bissantz C, Dehmlow H, Martin RE, Obst SU, Richter H, Ullmer Christoph. Novel phenyl amide or pyridyl amide derivatives. US2010/105906[P], 2010, A1, Page/Page column 48.
You may like
Related articles And Qustion
Lastest Price from METHYL OXALYL CHLORIDE manufacturers
Methyl Oxalyl Choride

US $6.00/kg2025-04-21
- CAS:
- 5781-53-3
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000
METHYL OXALYL CHLORIDE

US $0.00-0.00/KG2025-03-14
- CAS:
- 5781-53-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt