What is 4-Hydroxyindole?
Feb 19,2020
4-Hydroxyindole (1H-INDOL-4-OL; 1H-INDOLE-4-OL; 4-HYDROXY-1H-INDOLE; 4-INDOLOL; 4-HYDROXYINDOLE; INDOL-4-OL; 4-Hydroxy Indole (4-Indolol); C8H7NO) as simple indoles is the important raw material or intermediate in the synthesis of pharmaceutical products and industrial polymers [1]. Different approaches were proposed to realize preparing 4-hydroxyindole simply and efficiently.
Beer et al. developed a common strategy for preparing 4-hydroxyindole by using 6-nitrosalicylaldehyde as starting material [2]. In first, the condensation of 6-nitrosalicylaldehyde and nitromethane was accomplished in alcoholic potash at 0 oC, and the obtained alcohol was treated by hot acetic anhydride and sodium acetate, and converted into acetoxystyrene with a satisfactory yield. The acetoxystyrene was further reduced by iron filings and acetic acid and produced the 4-acetoxyindole, which would be transformed into the target product of 4-hydroxyindole by the hydrolysis with aqueous-methanolic sodium hydroxide in the presence of a mild reducing agent, sodium hydrosulphite. Recently, a useful method for preparing 4-hydroxyindole by ring-opening cyclization of cyclohexane-1,3-dione-2-spirocyclopropane with amine [2]. The synthesis procedure was showed in Fig.1. Firstly, 1,3-cyclohexanedionesl was reacted with diazo compound to produce 2-diazo-1,3-cyclohexanedione. Then, the obtained 2-diazo-1,3-cyclohexanedionean reacted with excess amount of styrene using a catalytic amount of Rh2(OAc)4 and gave spirocyclopropane. Finally, by ring-opening cyclization of spirocyclopropanewith amines, the high yields of tetrahydroindol-4(5H)-ones were obtained, one of which was easily converted to 4-hydroxyindole. In this procedure, the route to spirocyclopropanes from 1,3-cyclohexanediones could be also accomplished by reacting with using (1-aryl-2-bromoethyl)-dimethylsulfonium bromides, efficiently.
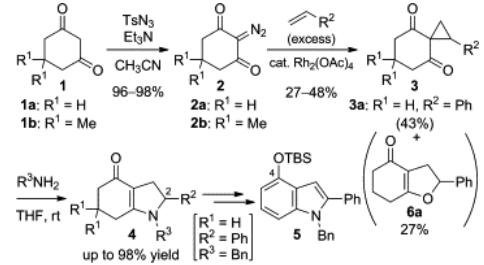
Fig.1 Synthesis of 4-hydroxyindole employing a ring-opening cyclization.
The 4-hydroxyindole also could be synthesized by a two-step process, including the palladium catalyzed cross-coupling of ortho-iodoanilines and (trimethylsilyl)acetylene, and a cyclization in the presence of potassium tert-butoxide [4]. Moreover, a palladium-catalyzed cyclization reaction of ortho-vinyl anilines has also been proposed to yield 4-hydroxyindole [5]. 4-Hydroxytryptamine scaffold was also successfully prepared in three steps with a good yield, including palladium-catalyzed cyclization of protected N-tert-butoxycarbonyl-2-iodo-3-methoxyaniline and an appropriately substituted silyl acetylene, and removal of the protecting groups.
Photochemical and electrochemical synthesis of 4-hydroxyindole have also been developed. In photochemical synthesis procedure, 5-(alkoxycarbonylamino)isoquinoline 2-oxides were irradiated in an aprotic solvent and treated with acid under solvolytic conditions that would produce 1-alkoxycarbonyl-4-hydroxyindoles 25% yields. 1-Benzyloxycarbonyl-4-hydroxyindole was then converted to the 4-hydroxyindole by catalytic hydrogenation [7]. In electrochemical synthesis procedure, 4-hydroxyindole were prepared via electrochemical oxidative coupling of 1,3-cyclohexadione with ethyl vinyl ether, and further ammonolysis and dehydrogenation [8].
In conclusion, 4-hydroxyindole as a useful intermediate for various chemical products, could be synthesized by different efficient routs, and is worthy of further study and optimize.
References
[1] https://www.chemicalbook.com/ProductChemicalPropertiesCB5663260.htm.
[2] Beer, R. J. S., Clarke, K., Khorana, H. G., & Robertson, A. (1948). 452. The chemistry of the melanins. Part I. The synthesis of 5: 6-dihydroxyindole and related compounds. Journal of the Chemical Society (Resumed), 2223-2226.
[3] Nambu, H., Fukumoto, M., Hirota, W., Ono, N., & Yakura, T. (2015). An efficient synthesis of cycloalkane-1, 3-dione-2-spirocyclopropanes from 1, 3-cycloalkanediones using (1-aryl-2-bromoethyl)-dimethylsulfonium bromides: Application to a one-pot synthesis of tetrahydroindol-4 (5H)-one. Tetrahedron letters, 56(29), 4312-4315.
[4] Aldrich: 4-benzyloxyindole (1 g), $A225; 4-hydroxyindole (1 g), $A288 (1/2003). A patent describing an efficient synthesis of 4-hydroxyindoles from cyclohexane-1,3-dione, where the key step is the reaction of oxochromancarboxylic acid derivatives with ammonia in methanol in an autoclave, has been filed. The author notes that this may effect the price of 4-hydroxyindoles in the future. Matsuura, T. (Nippon Zeon Co., Ltd., Japan) Jpn. Kokai Tokkyo Koho JP 2000044555, 2000.
[5] Kondo, Y., Kojima, S., & Sakamoto, T. (1997). General and facile synthesis of indoles with oxygen-bearing substituents at the benzene moiety. The Journal of Organic Chemistry, 62(19), 6507-6511.
You may like
Application research of 1-Bromohexadecane
Jan 30, 2026
Lastest Price from 4-Hydroxyindole manufacturers
4-Hydroxyindole

US $13.00-7.00/kg2024-08-20
- CAS:
- 2380-94-1
- Min. Order:
- 1kg
- Purity:
- 99.9%
- Supply Ability:
- 200ton
4-Hydroxyindole
US $122.00-1.00/KG2024-03-25
- CAS:
- 2380-94-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available