Methyl oxalyl chloride: properties, applications and safety
General Description
Methyl oxalyl chloride is a colorless to light yellow liquid with a pungent odor. It's a highly reactive acylating agent used in organic synthesis, including the production of pharmaceuticals and agrochemicals. Its polarity makes it soluble in organic solvents but insoluble in water. The compound's sensitivity to moisture necessitates anhydrous handling conditions. A notable application is its use in creating dolutegravir intermediates for HIV medication production. However, it poses significant safety concerns due to its corrosive nature and volatility. It can cause severe burns and eye damage, respiratory and digestive tract irritation, and it reacts vigorously with water, producing toxic gases. Therefore, proper PPE, ventilation, and safety procedures are essential when handling this compound.

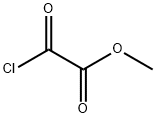
Figure 1. Methyl oxalyl chloride
Properties
Methyl oxalyl chloride is a colorless to light yellow liquid, chemically denoted as C3H3ClO3, with a distinct, sharp, and pungent odor. It has a molecular weight of about 122.51 g/mol. The compound is known for its high reactivity, which makes it a valuable acylating agent widely used in organic synthesis. This includes the preparation of pharmaceuticals and agrochemicals, where its reactivity plays a significant role. Methyl oxalyl chloride exhibits polarity, which contributes to its solubility in most organic solvents, although it is insoluble in water. A key property of this compound is its sensitivity to moisture. It can undergo hydrolysis when exposed to moisture, releasing hydrochloric acid, a corrosive and potentially hazardous substance. Therefore, it necessitates handling under strictly anhydrous conditions to prevent any undesired reactions. This careful handling underscores the importance of understanding its properties for safe and effective use. 1
Applications
Methyl oxalyl chloride is a versatile chemical reagent with a broad range of applications, particularly in the synthesis of pharmaceutical intermediates. One notable application is its role in a novel process for creating key dolutegravir intermediates, which are crucial components in the manufacture of HIV medications. In this method, methyl oxalyl chloride is condensed with ethyl 3-(N,N-dimethylamino)acrylate to yield a vinylogous amide with excellent efficiency. This compound is then substituted by aminoacetaldehyde dimethyl acetal and methyl bromoacetate, forming the precursor for cyclization. The cyclization process is facilitated by MgBr2, resulting in a highly selective conversion into pyridinone diester. The final step involves the selective hydrolysis of the diester using LiOH to produce the desired dolutegravir intermediate. Thus, methyl oxalyl chloride proves instrumental in this innovative synthesis pathway, showcasing its utility in complex organic transformations. 2
Safety
Methyl oxalyl chloride is a powerful acylating agent, characterized by its high reactivity and volatility. It's important to stress that this compound poses significant safety hazards, requiring meticulous handling and storage procedures. Firstly, it is a corrosive substance, which means it can cause severe burns and eye damage if it comes into direct contact with the skin or eyes. Therefore, it is essential to wear appropriate personal protective equipment (PPE), such as gloves, safety goggles, and lab coats when handling this compound. Secondly, inhalation or ingestion of methyl oxalyl chloride can lead to serious health complications, including irritation of the respiratory and digestive tracts. In an enclosed environment, ensure proper ventilation to minimize the risk of inhalation. If ingested, do not induce vomiting; instead, seek immediate medical attention. Moreover, methyl oxalyl chloride reacts vigorously with water, producing toxic gases. Consequently, it must be stored in a dry, well-ventilated area away from moisture. It's also flammable, necessitating strict control over potential ignition sources. In case of accidental spillage, it should be absorbed with an inert material like sand or vermiculite, then collected and disposed of according to local regulations. Lastly, emergency procedures should be in place for accidental exposure. This includes immediate rinsing of the affected area with plenty of water and seeking medical attention right away. 3
Reference
1. PubChem. COMPOUND SUMMARY: Methyl chloroglyoxylate. National Library of Medicine, 2005, PubChem CID: 79846.
2. Kong J, Xia H, He R, Chen H, Yu Y. Preparation of the Key Dolutegravir Intermediate via MgBr2-Promoted Cyclization. Molecules. 2021 May 11;26(10):2850.
3. Methyl chloroglyoxylate. European Chemicals Agency, EC / List no. 227-307-4.
Related articles And Qustion
See also
Lastest Price from METHYL OXALYL CHLORIDE manufacturers

US $6.00/kg2025-04-21
- CAS:
- 5781-53-3
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000

US $0.00-0.00/KG2025-03-14
- CAS:
- 5781-53-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt