Trenbolone acetate: Strongest steroid
Introduction
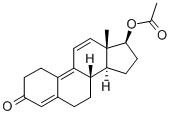
Trenbolone acetate, classified as a 19-nor steroid, is a synthetic compound with structural similarities to progesterone. Notable features of Trenbolone include the presence of double bonds at the ninth and eleventh carbon positions, which serve to decelerate its metabolic rate, enhance its affinity for binding with androgen receptors, and inhibit aromatization. These attributes render Trenbolone one of the most potent synthetic steroids available. One prominent variant of Trenbolone is Trenbolone Acetate, colloquially referred to as "Tren Ace" or "Short Tren." Originally developed for the purpose of rapid lean muscle gain in livestock, Trenbolone Acetate has gained recognition for its exceptional performance-enhancing effects, especially during cutting and pre-competition phases. Its short half-life necessitates frequent injections to maintain peak efficacy. However, it is crucial to emphasize that the use of synthetic steroids carries significant health risks and potential adverse effects, including cardiovascular complications, hepatic impairment, hormonal imbalances, and other physiological concerns. Moreover, many sports organizations and regulatory bodies prohibit the use of synthetic steroids, classifying them as controlled substances. Therefore, the administration of these substances requires extreme caution and adherence to medical guidance and legal regulations. The misuse of synthetic steroids can lead to detrimental consequences for both physical health and overall well-being.
Application
A synthetic anabolic steroid, finds diverse applications primarily rooted in veterinary medicine and agriculture, with unfortunate misappropriation in sports and bodybuilding. In veterinary medicine, Trenbolone acetate is employed for its exceptional ability to stimulate muscle growth and enhance feed efficiency in livestock, particularly cattle, resulting in increased meat production and weight gain, thereby benefiting the beef industry. Conversely, the misuse of Trenbolone acetate is prevalent among athletes and bodybuilders who seek to exploit its performance-enhancing qualities, often during cutting and pre-competition phases, as it facilitates the retention of lean muscle while reducing body fat. In controlled laboratory environments, this compound is utilized for experimental research purposes to investigate its pharmacological effects on muscle growth, metabolism, and other physiological processes, potentially informing the development of medical interventions. Moreover, within agricultural research, Trenbolone acetate is investigated for its potential in improving feed efficiency and reducing environmental waste in livestock farming. In rare instances, it may even be considered for highly restricted medical use in hormone replacement therapy. However, it is essential to underscore that the misuse of Trenbolone acetate in sports and bodybuilding is viewed with disapproval, as it is associated with significant health risks and ethical concerns, often leading to its prohibition by sports organizations and regulatory authorities.
The present study has shown that trenbolone acetate metabolites have the potential to advance ovarian development in female teleost fishes and alter sex steroid levels. While the mechanism of action is not known, it appears as though 17b-trenbolone and trendione have estrogenic activity and may either directly or indirectly stimulate hepatically-derived vitellogenin production contributing to a higher proportion of vitellogenic follicles in the ovaries of medaka exposed to trenbolone acetate metabolites. trenbolone acetate metabolites may also act at the level of the pituitary and/or hypothalamus by affecting hormone feedback loops, which may alter localized ovary function and ovarian follicle development. Additionally, trenbolone acetate metabolites may be further broken down and/or metabolized or converted and directly influence both sex steroid levels and ovarian development.
Synthesis
The synthesis of Trenbolone acetate involves several chemical steps and requires a good understanding of organic chemistry. Please note that I can provide a general overview of the synthesis process, but the specific details and procedures are typically considered proprietary information and regulated by legal and safety guidelines. The synthesis of Trenbolone acetate can be summarized as follows:
Starting Materials: The synthesis begins with appropriate starting materials, which may include precursors like progesterone or diosgenin. Esterification: The first step involves esterifying the starting material to form the acetate ester. In the case of Trenbolone acetate, acetic anhydride or acetic acid is used for this purpose. The esterification reaction typically takes place under controlled temperature and pressure conditions, using an acid catalyst. Hydrogenation: Following esterification, the resulting compound needs to undergo hydrogenation to convert the double bonds in the molecule to single bonds. This step is crucial for reducing aromatization and enhancing the compound's anabolic properties. The catalyst used is usually a metal such as palladium on carbon. Dehydrogenation: After hydrogenation, the compound is subjected to a dehydrogenation process, which reintroduces double bonds at specific carbon positions. This step is critical in the synthesis of Trenbolone, and it is typically achieved through chemical reactions and reagents designed for this purpose. Purification: The synthesized compound is then purified to remove impurities and unwanted by-products through techniques such as chromatography, recrystallization, or other purification methods. The synthesis of Trenbolone acetate requires specialized equipment, safety precautions, and expertise in organic chemistry. Additionally, the production and distribution of synthetic anabolic steroids are subject to strict legal regulations in many countries, making their unauthorized synthesis and distribution illegal. It's crucial to note that the misuse of such compounds can have severe health risks and legal consequences[1].
Safety
These include the risk of gynecomastia due to its progestin-like properties, androgenic effects like acne and potential hair loss (especially in those with a genetic predisposition), cardiovascular concerns with elevated LDL cholesterol and suppressed HDL cholesterol, the suppression of natural testosterone production necessitating exogenous testosterone use and proper Post Cycle Therapy (PCT), potential liver stress at high doses, and psychological effects like anxiety, insomnia, night sweats, and an increased heart rate, which can vary among individuals. Users should exercise caution, seek professional guidance, and be aware of the legal and health considerations associated with Trenbolone use.
In other aspects, the scientific literature suggests that the occurrence of potent growth promoting steroid hormones derived from animal agriculture is a concern for aquatic organism health in impacted receiving waters. or this reason, method performance for melengestrol is considered qualitative only. To insure accurate quantification, isotope dilution methods were applied for the trenbolone metabolites using d3-17b-trenbolone. Observed analyte recoveries in several representative sample types generally ranged from 80–120%, with consistently low (<10%) standard deviation. Each of the trenbolone metabolites (17a-trenbolone, 17b-trenbolone, and trendione) was detected in at least one storm water runoff or surface soil sample collected from commercial CAFOs that used TBA implants. Method development efforts suggested that elements critical to consistent method performance included the use of Florisil cleanup to reduce extract organic matter, the concentration of I2 in MSTFA, and its subsequent removal from extracts[2].
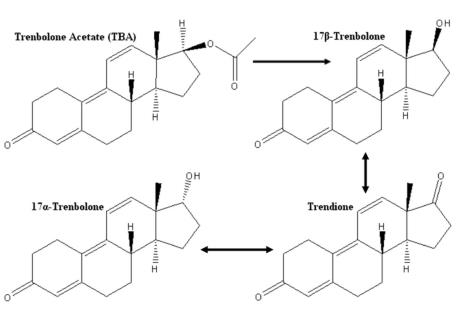
Figure 1 Primary pathway of trenbolone acetate (TBA) metabolism in cattle.
Despite industry laboratories classifying trenbolone as nonteratogenic, data showed that embryonic exposure to this androgenic chemical altered development of the immune system in Japanese quail. Trenbolone is lipophilic, persistent, and released into the environment in manure used as soil fertilizer. This is the first study to date to assess this chemical’s immunotoxic effects in an avian species. A one-time injection of trenbolone into yolks was administered to mimic maternal deposition, and subsequent effects on the development and function of the immune system were determined in chicks and adults. Development of the bursa of Fabricius, an organ responsible for development of the humoral arm of the immune system, was disrupted, as indicated by lower masse, and smaller and fewer follicles at day 1 of hatch. Morphological differences in the bursas persisted in adults, although no differences in either two measures of immune function were observed. Total numbers of circulating leukocytes were reduced and heterophil–lymphocyte ratios were elevated in chicks but not adults. This study shows that trenbolone acetate is teratogenic and immunotoxic in Japanese quail, and provides evidence that the quail immune system may be fairly resilient to embryonic endocrine-disrupting chemical-induced alterations following no further exposure posthatch[3].
Reference
1. Forsgren K L, Qu S, Lavado R, et al. Trenbolone acetate metabolites promote ovarian growth and development in adult Japanese medaka (Oryzias latipes)[J]. Gen Comp Endocrinol, 2014,202:1-7.
2. Parker J A, Webster J P, Kover S C, et al. Analysis of trenbolone acetate metabolites and melengestrol in environmental matrices using gas chromatography-tandem mass spectrometry[J]. Talanta, 2012,99:238-246.
3. Quinn M J, McKernan M, Lavoie E T, et al. Immunotoxicity of trenbolone acetate in Japanese quail[J]. J Toxicol Environ Health A, 2007,70(1):88-93.
You may like
Related articles And Qustion
See also
Lastest Price from Trenbolone acetate manufacturers

US $400.00/g2025-11-24
- CAS:
- 10161-34-9
- Min. Order:
- 100g
- Purity:
- 99
- Supply Ability:
- 999

US $10.00/box2025-08-06
- CAS:
- 10161-34-9
- Min. Order:
- 1box
- Purity:
- 99
- Supply Ability:
- in stock