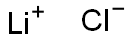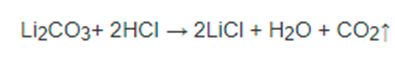What is a positive flame test for Lithium chloride?
Lithium Chloride is an ionic compound or salt that is highly polar and soluble in water. It is more soluble in organic solvents such as acetone and methanol than potassium chloride or sodium chloride.
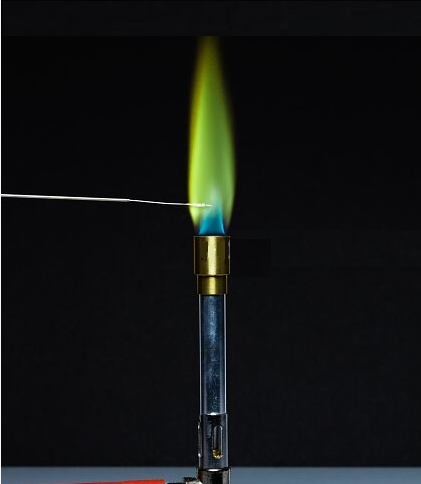
Lithium chloride flame test

The flame test is an analytical chemistry method used to help identify metal ions.
The flame color of lithium chloride is a dark red flame. This is due to the excitation of electrons in the lithium by the heat of the flame. As these electrons lose their energy, they emit photons of red light. The colour of the flame is different for different elements and can be used to identify unknown substances.
Uses
Lithium chloride is mainly used for the production of lithium metal by electrolysis of a LiCl/KCl melt at 450°C (842 °F). LiCl is also used as a brazing flux for aluminium in automobile parts. It is used as a desiccant for drying air streams.
You may like
Related articles And Qustion
See also
Lastest Price from Lithium chloride manufacturers

US $0.00/KG2025-04-21
- CAS:
- 7447-41-8
- Min. Order:
- 25KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $1.00/KG2025-04-21
- CAS:
- 7447-41-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt