Barium: Discovery, Sources and the Use in Fireworks
The Discovery of Barium
The Swedish chemist Carl Wilhelm Scheele discovered (1774) a new base (baryta, or barium oxide, BaO) as a minor constituent in pyrolusite, and from that base he prepared some crystals of barium sulfate, which he sent to Johan Gottlieb Gahn, the discoverer of manganese. A month later Gahn found that the mineral barite is also composed of barium sulfate, BaSO4. A particular crystalline form of barite found near Bologna, Italy, in the early 17th century, after being heated strongly with charcoal, glowed for a time after exposure to bright light. The phosphorescence of "Bologna stones" was so unusual that it attracted the attention of many scientists of the day, including Galileo. Only after the electric battery became available could Sir Humphry Davy finally isolate (1808) the element itself by electrolysis.
The Sources of Barium
In nature, Barium is always found combined with other elements. Barium constitutes about 0.03 percent of Earth's crust, chiefly as the minerals barite (also called barytes or heavy spar) and witherite. Between six and eight million tons of barite are mined every year, more than half of it in China. Lesser amounts are mined in India, the United States, and Morocco. Commercial production of barium depends upon the electrolysis of fused barium chloride, but the most effective method is the reduction of the oxide by heating with aluminum or silicon in a high vacuum. A mixture of barium monoxide and peroxide can also be used in the reduction. Only a few tons of barium are produced each year.
Barium and Fireworks
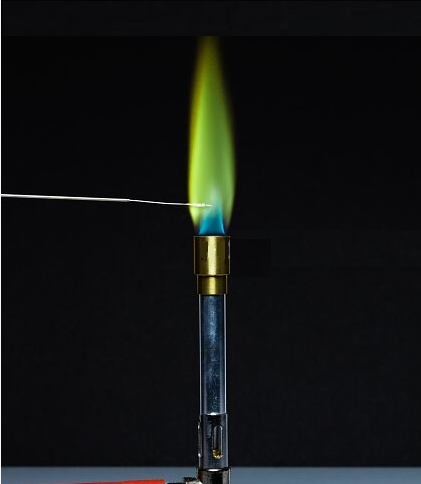
Flame colors are produced from the movement of the electrons in the metal ions present in the compounds. Each different metal will have a different pattern of spectral lines, and so a different flame color. Barium emits a green flame when it burns, producing barium peroxide. Therefore, people use this property of Barium (the flame color) to create the green effect in fireworks. The method is to mix barium nitrate with potassium chlorate, magnesium powder, and rosin to create flares and fireworks.