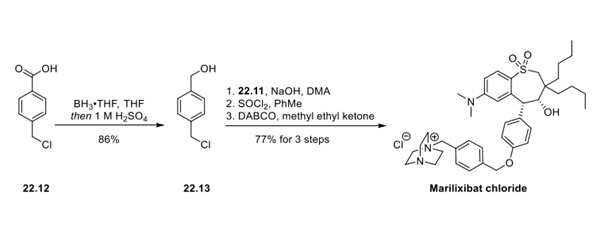
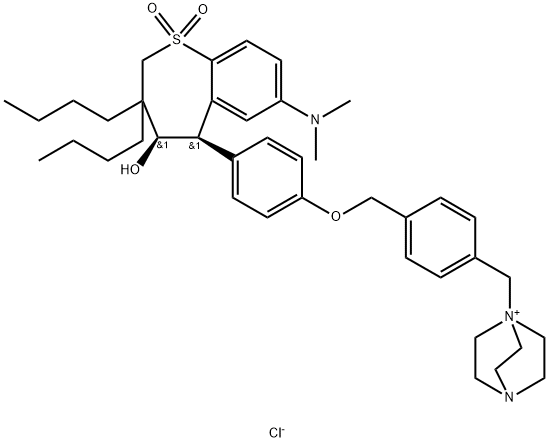
How is Marilixibat Chloride synthesised?
Synthesis of Marilixibat Chloride
Marilixibat Chloride was synthesised by the reaction of two intermediate compounds with diethyl dibutylmalonate as the starting material. The specific synthesis steps are as follows:
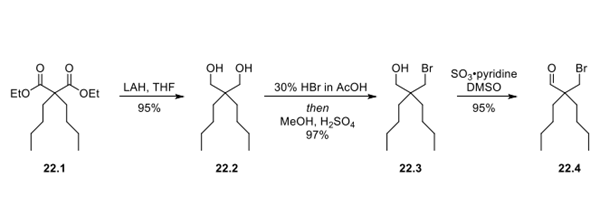
Step 1: Synthesis of Bromoaldehyde Intermediate 22.4
First, diethyl dibutylmalonate (22.1)
was reduced to diol 22.2 with lithium aluminum hydride in 95%
yield. Next, the diol was carefully converted to
bromoalcohol (22.3) with HBr in acetic acid. Finally, the
alcohol was oxidized to bromoaldehyde (22.4) with a sulfur
trioxide pyridine complex.
Step 2: Synthesis of Intermediate 22.11
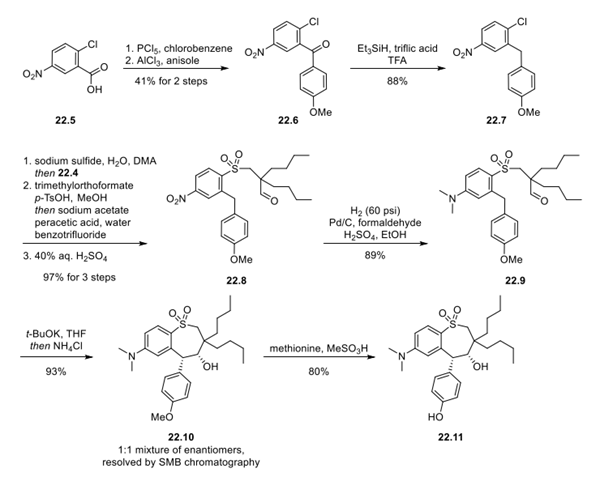
Next, 2-chloro-5-nitrobenzoic acid (22.5) was converted to the acid chloride with phosphorus pentachloride, and the resulting product was used directly in a Friedel−Crafts acylation reaction with anisole to deliver ketone 22.6. Deoxygenation of 22.6 was achieved by using triethylsilane and triflic acid in TFA to provide arene 22.7. Next 22.7 was treated with sodium sulfide, resulting in an SNAr displacement of chloride; intermediate 22.4 was then added to the reaction, resulting in thioether formation via SN2 displacement of the bromide (22.4). The aldehyde of the thioether was protected as a dimethyl acetal prior to oxidation of the thioether to the sulfone with sodium acetate and peracetic acid in one pot. Finally, the acetal was hydrolyzed back to the aldehyde to deliver 22.8 in 97% yield over 3 steps. Catalytic hydrogenation in the presence of formaldehyde and sulfuric acid converted the nitro group of 22.8 to the dimethylamino group, delivering 22.9 in 89% yield. Treatment of 22.9 with potassium t-butoxide in THF resulted in a thermodynamically controlled stereoselective cyclization that set the hydroxyl and methoxyphenyl groups of 22.10 in a syn relationship. The cyclized product (22.10) was isolated in 93% yield as a 1:1 mixture of enantiomers, which were separated by simulated moving bed (SMB) chromatography. Conditions were provided for racemization of the undesired 4S,5S enantiomer using potassium tert-butoxide, suggesting that this could be resubjected to SMB chromatography to improve throughput. The purified 4R,5R-22.10 was treated with methionine and methanesulfonic acid to provide phenol 22.11 in 80% yield.
Step 3: Completion of the Marilixibat Chloride Synthesis
4- chloromethylbenzoic acid was reduced to benzyl alcohol (22.13) with a borane−THF adduct in 86% yield. Benzylic chloride 22.13 was then used in an SN2 reaction with phenol 22.11. The benzylic alcohol was then converted to the chloride, which was subsequently displaced by diazabicyclo[2.2.2]octane (DABCO) to deliver marilixibat chloride in 77% yield over 3 steps.