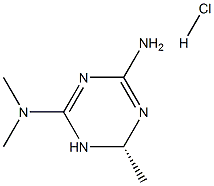
What is the synthetic pathway for Imeglimin hydrochloride?
Synthesis of Imeglimin hydrochloride
Imeglimin hydrochloride is synthesised using metformin hydrochloride as a raw material by chemical reaction. The specific synthesis steps are as follows:
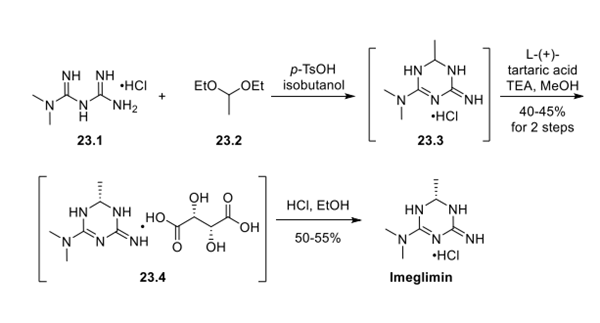
Treatment of metformin hydrochloride (23.1) with acetaldehyde diethyl acetal (23.2) under refluxing conditions in isobutanol and catalytic acid provided crude racemic 23.3. After concentration, crude 23.3 was treated with triethylamine and L-(+)-tartaric acid. The diastereomerically pure tartrate salt 23.4 was obtained in 40−45% yield over 2 steps after crystallization. Salt metathesis was accomplished by the treatment of 23.4 with ethanolic HCl. The enantiomerically pure product 23 was obtained in 50−55% yield after crystallization from cold EtOH.
Introduction of Imeglimin hydrochloride
Imeglimin hydrochloride, discovered by Poxel and co-developed with Sumitomo Dainippon Pharma Co., was approved for use in Japan as a key treatment for T2D. Imeglimin is the first approved tetrahydrotriazine-containing class of oral antidiabetic agents known as the glimins. Imeglimin has been shown to protect mitochondrial function from oxidative stress in a high-fat, highsucrose diet mouse model, resulting in a significant reduction of glycemia, restored glucose tolerance, and improved insulin sensitivity. Imeglimin has also been shown to increase glucose uptake within the skeletal muscle and inhibit gluconeogenesis in the liver to a similar level as metformin.
You may like
See also
US $0.00/KG2025-09-29
- CAS:
- 775351-61-6
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1000KG