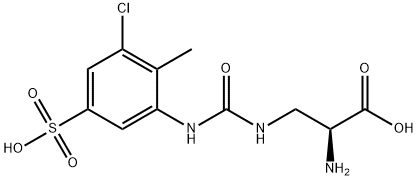
Upacicalcet: Synthesis and Introduction
Synthesis of Upacicalcet
Upacicalcet is synthesised using aniline as a raw material by chemical reaction. The specific synthesis steps are as follows:
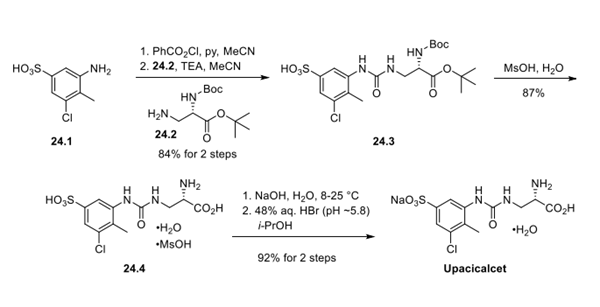
Aniline 24.1 was subjected to phenyl chloroformate prior to treatment with amine 24.2 and a base to furnish urea 24.3 in excellent conversion. Methanesulfonic acid-mediated removal of Boc and the tert-butyl ester resulted in the formation of hydrated mesylate salt 24.4. Treatment of this sulfonic acid with sodium hydroxide followed by careful isopropanolic pH adjustment afforded upacicalcet as the hydrated sodium salt.
Introduction of Upacicalcet
Upacicalcet is an intravenously administered calcimimetic agent developed by Sanwa Kagaku Kenkyusho, under license from EA Pharma, for the treatment of secondary hyperparathyroidism (SHPT), a common and early complication of CKD, in patients undergoing hemodialysis. By acting directly on parathyroid cell membrane calcium-sensing receptors, the drug suppresses excessive parathyroid hormone (PTH) secretion, thereby lowering blood PTH levels. PTH regulates the calcium concentration in the blood plasma to maintain calcium homeostasis in the body tissues-a concept often termed "calcium balance". Upacicalcet received its first approval in 2021 for the treatment of SHPT in adults undergoing hemolysis in Japan. It is administered intravenously three times per week into the venous side of the hemodialysis circuit at the time of blood return following the end of the hemodialysis session.