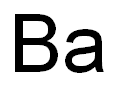Exploring the Significance of Barium Chemistry in Soil Distribution and Plant Uptake
General Description
The field of barium chemistry focuses on studying the properties and behavior of the element barium and its compounds. Barium is highly reactive and exists mainly as the Ba2+ cation in various compounds. Understanding barium chemistry is crucial for environmental impact assessment, chemical reactions, and industrial applications. In soils, barium distribution can be affected by the presence of chemical fertilizers, and its retention on clay minerals is influenced by factors like soil acidification and competition with other ions. Barium uptake by plants primarily occurs through natural sources, such as seawater and the continental crust. It exhibits an affinity for plants, is actively transported through roots, and can be translocated to leaves or needles via the xylem. The depth of plant roots and biogeochemical cycling processes play important roles in the enrichment of barium in topsoil. Overall, understanding barium chemistry is essential for comprehending its role in soils and plants and its potential environmental implications.

Figure 1. Barium
Chemistry
Barium chemistry involves the study of the element barium (Ba), which is a member of group IIA in the Periodic Table. Barium is characterized by its silvery-white appearance, soft texture, and susceptibility to oxidation by air and water due to its high reactivity. As a result, barium is not found in its elemental form in nature but exists predominantly as the Ba2+ cation in compounds such as barite (BaSO4) and witherite (BaCO3). Various barium salts, including acetate, chloride, sulfide, and nitrate, along with compounds like barium hydroxide, are derived from barite and exhibit different levels of solubility in water. While natural sources of barium do not pose significant risks to drinking water contamination, concerns arise from anthropogenic activities leading to barium pollution in the environment. The diverse properties and behaviors of barium compounds make the field of barium chemistry crucial for understanding environmental impacts, chemical reactions, and industrial applications involving this element. 1
Barium in soils and plants
Barium in soils
Barium, with an estimated crustal abundance of approximately 425 ppm, ranks as the 14th most abundant element in the Earth's crust. This metal is relatively common in ordinary rocks compared to other elements like manganese. Barium in soil can be influenced by the presence of chemical fertilizers rich in potassium (K+) and/or ammonium (NH4+), which can mobilize Ba2+ ions previously adsorbed by clay minerals in the soil. Competition for binding sites on clay minerals' surfaces occurs between K+ or NH4+ and Ba2+, affecting the distribution of these ions. Moreover, Ba2+ is strongly adsorbed by clay minerals due to its similar ionic radius to K+, allowing it to occupy similar binding positions. The electrostatic attraction force of Ba2+ to the clay mineral surface is significantly higher than that of K+, aiding in its retention. The concentration ratios of elements like rubidium (Rb) and potassium (K) in soils can provide insights into natural soil-forming processes or anthropogenic disturbances such as pollution. Factors like soil acidification from fertilizer applications can impact the release of tightly bound cations like K+, NH4+, and Ba2+. Additionally, the retention of Ba2+ on clay minerals in the topsoil may be influenced by preferential leaching of K+ or lower uptake of K+ compared to Ba2+ by plant roots, leading to variations in the Barium/K concentration ratio over time. In summary, understanding the dynamics of barium in soil involves considering the interactions with other elements, soil properties, and anthropogenic activities to assess its distribution and potential environmental implications. 2
Barium uptake by plants
The uptake of barium by plants predominantly originates from natural sources, such as seawater and the continental crust, rather than commercial fertilizers. Barium's low concentration in phosphorites and the limited solubility product of barium sulfate in seawater further support this notion. Studies indicate that barium exhibits an affinity for plants, as evidenced by its higher concentration in plant leaves compared to zinc, a commonly abundant element in the continental crust. Barium is actively transported through plant roots and then translocated to leaves or needles via the xylem. The depth of plant roots and the biogeochemical cycling process influence the enrichment of barium in topsoil. Additionally, if the rate of barium uptake by plant roots exceeds its removal from the topsoil, the concentration ratio of barium to potassium in the topsoil may increase until reaching a steady state. Furthermore, the chemical similarities between barium and radium suggest that their uptake by plant roots may be similar if soluble uranium is present in the soil. This comprehensive understanding underscores the significance of natural sources and the intricate processes involved in the uptake and cycling of barium within plant ecosystems. 3
Reference
1. Peana M, Medici S, Dadar M, et al. Environmental barium: potential exposure and health-hazards. Arch Toxicol. 2021; 95(8): 2605-2612.
2. Nan XY, Yu HM, Roberta L. Barium isotopic composition of the upper continental crust. Geochimica et Cosmochimica Acta. 2018; 233: 33-49.
3. Pyle K, Hendry K, Sherrell R, Meredith M, Venables H, Lagerström M, Morte-Rodenas A. Coastal barium cycling at the West Antarctic Peninsula. Deep Sea Res Part II Top Stud Oceanogr. 2017; 139: 120–131.