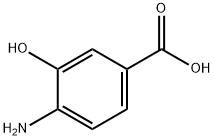
What is 4-Amino-3-hydroxybenzoic acid?
4-Amino-3-hydroxybenzoic acid is used in the preparation of various pharmaceutical compounds such as sphingosine kinase inhibitors. Store at 4℃. Store in cool, dry conditions in well-sealed containers. Keep container tightly closed. Store away from strong oxidizing agents.
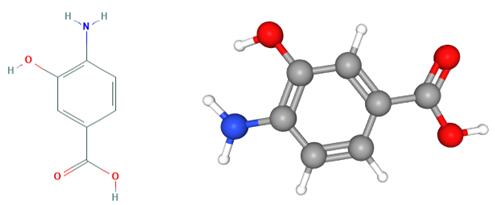
Fig 1. Chemical structure formula and three-dimensional structure of 4-Amino-3-hydroxybenzoic acid
4-Amino-3-hydroxybenzoic acid is used in the preparation of various pharmaceutical compounds such as sphingosine kinase inhibitors[1].Bordetella sp. strain 10d metabolizes 4-amino-3-hydroxybenzoic acid via 2-hydroxymuconic 6-semialdehyde. Cell extracts from 4-amino-3-hydroxybenzoate-grown cells showed high NAD+-dependent 2-hydroxymuconic 6-semialdehyde dehydrogenase, 4-oxalocrotonate tautomerase, 4-oxalocrotonate decarboxylase, and 2-oxopent-4-enoate hydratase activities, but no 2-hydroxymuconic 6-semialdehyde hydrolase activity. These enzymes involved in 4-amino-3-hydroxybenzoate metabolism were purified and characterized. When 2-hydroxymuconic 6-semialdehyde was used as substrate in a reaction mixture containing NAD+ and cell extracts from 4-amino-3-hydroxybenzoate-grown cells, 4-oxalocrotonic acid, 2-oxopent-4-enoic acid, and 4-hydroxy-2-oxovaleric acid were identified as intermediates, and pyruvic acid was identified as the final product. A complete pathway for the metabolism of 4-amino-3-hydroxybenzoic acid in strain 10d is proposed. Strain 10d metabolized 2-hydroxymuconic 6-semialdehyde derived from 4-amino-3-hydroxybenzoic acid via a dehydrogenative route, not via a hydrolytic route. This proposed metabolic pathway differs considerably from the modified meta-cleavage pathway of 2-aminophenol and those previously reported for methyl- and chloro-derivatives.
2-Amino-5-carboxymuconic 6-semialdehyde is an unstable intermediate in the meta-cleavage pathway of 4-amino3-hydroxybenzoic acid in Bordetella sp. strain 10d. In vitro, this compound is nonenzymatically converted to 2,5-pyridinedicarboxylic acid. Crude extracts of strain 10d grown on 4-amino-3-hydroxybenzoic acid converted 2-amino-5-carboxymuconic 6-semialdehyde formed from 4-amino-3-hydroxybenzoic acid by the first enzyme in the pathway,4-amino-3-hydroxybenzoate 2,3-dioxygenase, to a yellow compound[3].
References
[1]Chika Orii; Shinji Takenaka; Shuichiro Murakami; and Kenji Aoki . A novel coupled enzyme assay reveals an enzyme responsible for the deamination of a chemically unstable intermediate in the metabolic pathway of 4-amino-3-hydroxybenzoic acid in Bordetella sp. strain 10d. Eur. J. Biochem. 2004, 271,(15), 3248-3254.
[2]Orii C , Takenaka S , Murakami S , et al. Metabolism of 4-amino-3-hydroxybenzoic acid by Bordetella sp. strain 10d: A different modified meta-cleavage pathway for 2-aminophenols.[J]. Bioscience Biotechnology & Biochemistry, 2006, 70(11):2653-2661.
[3]Orii C . A novel coupled enzyme assay reveals an enzyme responsible for the deamination of a chemically unstable intermediate in the metabolic pathway of 4‐amino‐3‐hydroxybenzoic acid in Bordetella sp. strain 10d[J]. European Journal of Biochemistry, 2010, 271(15):3248-3254.
[4]https://pubchem.ncbi.nlm.nih.gov/compound/137566
[5]http://www.chemspider.com/Chemical-Structure.121227.html?rid=02512d5c-ee63-45e3-83e6-6355c54e11db
You may like
Related articles And Qustion
Lastest Price from 4-Amino-3-hydroxybenzoic acid manufacturers
US $0.00-0.00/kg2025-08-26
- CAS:
- 2374-03-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $0.00-0.00/KG2025-05-07
- CAS:
- 2374-03-0
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS