What is Diethyl chlorothiophosphate?
Dimethyl chlorothiophosphate is a chemical that is used as an intermediate in the manufacture of pesticides and plasticisers. It is an organophosphate with sulfur and chlorine also bonded to the central phosphorus atom. In 1985 American Cyanamid had an accidental release of this chemical from its Linden plant, and it was smelled 32 km away.Dimethyl chlorothiophosphate is incompatible with bases (including amines), with strong oxidizing agents, and with alcohols. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts[1,2].

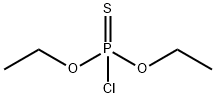
Fig 1. Chemical structure formula and three-dimensional structure of Diethyl chlorothiophosphate
Diethylthiophosphoryl chloride appears as a colorless to light amber liquid with a disagreeable odor. Insoluble in water but soluble in most organic solvents. May be highly toxic and irritating to the eyes and lungs.Used as an intermediate for pesticides, as an oil and gasoline additive, and as a flame retardant.
Diethylthiophosphoryl chloride (referred to ethyl chloride) are key intermediates in the production of organic pesticides; traditional production process production process generally vulcanized chloride, an alkali solution, washing with water, dehydration treatment , a large number of production process wastewater, wastewater with a high concentration, deep color, toxicity susceptible to biodegradation characteristics, mainly due to the alkaline hydrolysis process using alkali solution of an alkali solution of sodium sulfide and sodium hydroxide, then subjected to washing, produce large amounts of waste water per ton of product produced three tons of high strength wastewater, the wastewater after acidification, oxidation, desalination, chemical and biological treatment in order to discharge standards[1,4].
Adopting phosphorus pentasulfide as raw materials, conducting reaction between the phosphorus pentasulfide and absolute ethyl alcohol to obtain Diethylthiophosphoryl chloride (ethyl sulfide for short); then reacting with fixed quantity of chlorine gas to obtain ethyl chloride crude products; adding a catalyst, conducting post-chlorination reaction to obtain ethyl chloride and crystalloid sulphur, centrifugally separating the sulphur, and distilling by a film evaporator to obtain more than 99% of a high-purity product.
References
[1]Hiroyuki Kataoka, Seiko Shindoh, Masami Makita. Selective determination of volatile N-nitrosamines by derivatization with diethyl chlorothiophosphate and gas chromatography with flame photometric detection[J]. 723(1):93-99.
[2]K. CHATURVEDI, S. K. SRIVASTAVA, O. P. PANDEY. ChemInform Abstract: Organic Derivatives of Phosphorus as Pesticides. Part 2. Reactions of Diethyl Chlorothiophosphate with Substituted Mercapto Triazines[J]. Cheminform, 1995, 26(47).
[2]http://www.chemspider.com/Chemical-Structure.16375.html?rid=fb09df27-8091-4be2-a3a5-0c1e1f557811&page_num=0
[2]https://pubchem.ncbi.nlm.nih.gov/compound/17305

![3637-26-1 3-[Dimethyl-[2-(2-methylprop-2-enoyloxy)ethyl]azaniumyl]propane-1-sulfonate(DMAPS);Application; Use](httpss://www.chemicalbook.com/NewsImg/2020-1-16/20201161118316289.jpg)