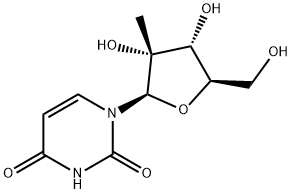
2'-C-Methyluridine:a modified nucleoside
Introduction
2'-C-Methyluridine is a modified nucleoside, specifically a modified form of uridine, one of the building blocks of RNA. In the structure of 2'-C-Methyluridine, a methyl group is added at the 2' carbon position of the ribose sugar in the nucleoside. Modifications to nucleosides, like 2'-C-Methyluridine, are common in nucleic acid chemistry and biology research. These modifications can affect the stability, binding affinity, and other properties of RNA molecules. Researchers use modified nucleosides for various purposes, including the study of RNA structure and function, as well as the development of therapeutic agents. The introduction of a methyl group at the 2' carbon position in this case can influence the conformation and interactions of the nucleoside within RNA molecules. It's important to note that specific details about the properties and uses of 2'-C-Methyluridine can depend on the context of its application, such as whether it's being used in the laboratory for research purposes or in the development of therapeutic agents.
Application
The application of 2'-C-Methyluridine, like many modified nucleosides, can vary depending on the specific goals of research or development. Here are a few common applications: RNA Structure and Function Studies: Researchers use modified nucleosides to study the structure and function of RNA molecules. The introduction of a methyl group at the 2' carbon position in 2'-C-Methyluridine can alter the conformation and interactions within RNA, providing insights into the role of specific nucleotides in RNA folding and stability. Antiviral Drug Development: Modified nucleosides are sometimes explored for their potential therapeutic applications. In the case of 2'-C-Methyluridine, it has been investigated for antiviral properties. Some nucleoside analogs can interfere with the replication of viruses, making them potential candidates for the development of antiviral drugs. RNA-based Therapeutics: Modified nucleosides may play a role in the development of RNA-based therapeutic agents. The modifications can enhance the stability and pharmacokinetic properties of RNA molecules, improving their therapeutic potential. Chemical Biology: 2'-C-Methyluridine and similar modified nucleosides are valuable tools in chemical biology research. They can be used to probe RNA structure and function, as well as to develop new techniques for manipulating and studying nucleic acids. Biotechnology: Modified nucleosides find applications in biotechnology, particularly in the development of novel nucleic acid-based technologies and diagnostics.
The catalytic core of an 8-17 DNAzyme directed against STAT 3 was modified using (2'R) and (2'S) 2'-deoxy-2'-C-methyluridine and cytidine. While 2'-deoxy-2'-C-methyluridine significantly diminished the catalytic activity, 2'-deoxy-2'-C-methylcytidine replacement was better accepted, being the kactof modified DNAzymes at 8- and 11-positions comparable to the non-modified one. When 2'-O-methyl and phosphorothioate nucleotides were tested in the binding arms together with core modified DNAzymes the kcatwas affected in a non predictable way, emphasizing the fact that both chemical substitutions should be considered globally. Finally, 2'-deoxy-2'-C-methyl modified DNA zymes stability was assayed finding that the double 2'-C-methyl modification in the catalytic core enhanced 70% the stability against a T47D cell lysate compared to a non-modified control2. It's important to note that the specific application of 2'-C-Methyluridine can depend on the research or development context, and ongoing studies may uncover new applications or refine existing ones.
Synthesis
The development of synthetic oligoribonucleotides with non conventional activities, like catalytic ribozymes, aptamers and more recently siRNA, found applications in different fields such as therapeutics, biosensors and molecular biology. The susceptibility of RNA to the attack of nucleases limited its utilization, especially in medicine. A way to overcome this restriction is the use of chemical modifications, which must fulfill certain requisites. Another important feature is related to RNA structure, in this sense sugar puckering of modified nucleosides can play an importan role, like in the case of locked nucleic acids (LNA). (2'R)-2'-C-methylribonucleosides are attractive candidates to be used in modified RNA structures, because they showed increased nuclease resistance, when incorporated to RNA sequences; and can adopt C-3' endo (RNA-like) conformation with anti orientation of the base. An important attribute of 2'-C-methylribonucleosides is that they posses the 2'-hydroxyl. This functional group could be essential to mimic RNA polar interactions and hydrogen bonding of complex RNA tertiary structures. 2'-C-methyl nucleosides have been synthesized using two main strategies: (i) construction of the modified sugar moiety and posterior glycosidation reaction, and (ii) modification of the ribose portion of a natural ribonucleoside. Related to the first approach, glucose, fructose or ribose have been employed as starting materials making use of regioselective deprotection at position 211 or of alkaline sugar degradation1.
The direct modification of the ribose moiety of a natural ribonucleoside can be also accomplished by two different routes, both involving as a common synthon, the 2'-ketonucleoside. A new synthesis of 2'-C-Methyluridine is presented. The 2'-hydroxyl group of the ribose in uridine is selectively methylated. This can be achieved through reactions with methylating agents such as diazomethane or other methylating reagents. Special emphasis is dedicated to the improvement of the protection of the tertiary 2'-hydroxyl group. Comparison to previous protecting strategies and analysis of stability under 5'-DMTr removing conditions are discussed. The synthetic incorporation of this modified nucleoside into the catalytic core of a hammerhead ribozyme against the estrogen receptor alpha protein (ER-alpha), and transfection experiments in MCF-7 cell line are also presented1.
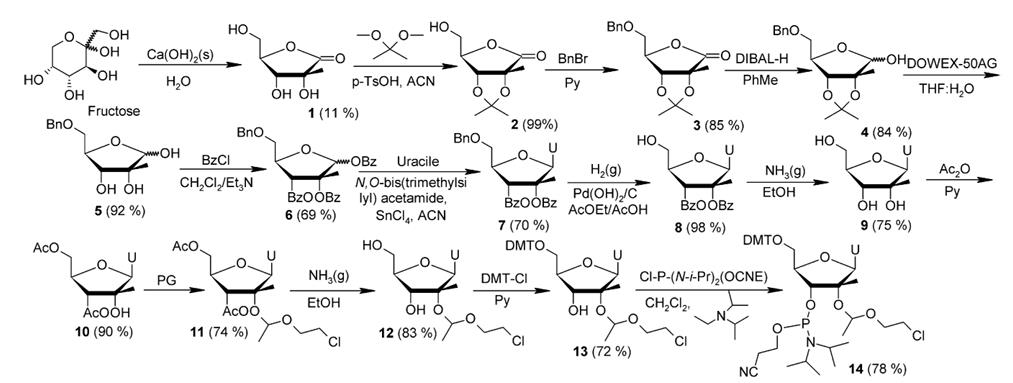
Figure 1. Synthesis of 2'-C-Methyluridine from fructose
Safety
When working with compounds like 2'-C-Methyluridine, ensuring safety is paramount. Personal Protective Equipment (PPE), including gloves, lab coat, safety glasses, and possibly a face shield, should be worn. Conduct work in a well-ventilated area, such as a fume hood, to minimize inhalation exposure, and adhere to proper handling and storage procedures, considering compatibility and designated storage areas. Familiarize yourself with emergency equipment locations and procedures. Proper training is essential, covering chemical properties, hazards, and emergency response. Conduct a thorough risk assessment, considering toxicity, flammability, and reactivity, and implement appropriate control measures. Dispose of waste in accordance with regulations and guidelines. Be prepared with first aid measures, and maintain accurate records of handling, storage, and disposal. Specific safety considerations may vary, so consult safety data sheets and adhere to institutional and regulatory guidelines throughout the process.
Reference
1. Dellafiore M, Avió A, Alagia A, Montserrat JM, Iribarren AM, Eritja R. siRNA Modified with 2'-Deoxy-2'-C-methylpyrimidine Nucleosides. Chembiochem. 2018 Jul 4;19(13):1409-1413.
2. Dellafiore M, Montserrat JM, Iribarren AM. Core modified 8-17 DNA zymes with 2'-deoxy-2'-C-methylpyrimidine nucleosides. Bioorg Chem. 2020 Nov;104:104328.
Related articles And Qustion
See also
Lastest Price from 2'-C-Methyluridine manufacturers

US $1.00/KG2019-07-06
- CAS:
- 31448-54-1
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1tons