D-glucuronic acid: production methods and application
Introduction
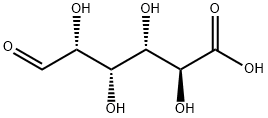
D-glucuronic acid (Figure 1, C6H10O7) is an important compound, which can be generated through the oxidation of the primary alcohol hydroxyl group of glucose into a carboxyl group. D-glucuronic acid widely exists in plants and animals in the form of tissue complexes. For example, chondroitin sulfate in human connective tissues and Callaya resin glue in plants are rich in D-glucuronic acid, Hyaluronic acid has excellent moisturizing properties and biocompatibility, it is a linear polysaccharide composed of, alternately linking N-acetyl-D-glucosamine and D-glucuronic acid. It is found mainly in connective tissue, skin, cartilage and eyeball of humanbody. It can absorb and lock in moisture, and each gram of hyaluronic acid can absorb at least 1000 ml of water,keeping the skin moist.[1]

Of the three naturally occurring hexuronic acids, D-glucuronic acid appears to be by far the most widely distributed. It has not been found free in Nature, except possibly in small amounts in blood and urine,but it occurs in a wide variety of polysaccharides and mucoproteins of plant, animal and bacterial origin, and plays an important part in the metabolism of many types of organic compounds. Sustained interest in D-glucuronic acid dates from about 1875 when von Mering and Musculus showed it to be a component of urochloralic acid, a metabolite of chloral hydrate, and Jaffe isolated it from a metabolite of o-nitrotoluene. Later Schmiedeberg and Meyer obtained D-glucurone from camphor-D-glucuronic acid,a metabolite of camphor. The structural relationship of D-glucuronic acid to D-glucose was established by Fischer and Piloty in 1891 when they obtained it by reduction of D-saccharic acid with sodium amalgam.[2]
Main production methods for D-glucuronic acid
At present, the main methods for D-glucuronic acid production include chemical oxidation, polysaccharide hydrolysis and biological catalysis. Chemical oxidation is the most commonly used for D-glucuronic acid production.Generally, starch is oxidised by concentrated nitric acid and hydrolyzed under high pressures. This method shows poor selectivity and the generation of byproducts, which leads to the low yield of D-glucuronic acid,and high energy consumption. With the continuous development of research, more clean and efficient large-scale production methods have been put into practice. For instance,Moon et al proposed to use sodium borohydride and hydrogen to replace concentrated nitric acid for oxidation reaction, which greatly improved the yield of D-glucuronic acid and glucuronolactone, the production has increased from 10 to 12% of domestic companies to over 20%.Biological catalysis is one of the most promising production methods of D-glucuronic acid. According to the production path from glucose to D-glucuronic acid, reactions can be divided into enzyme-catalyzed and whole-cell catalyzed ones. Enzyme catalysis is one of the most studied methods at present. Under the action of the intracellular enzyme myo-inositol oxidase and oxygen, myoinositol can be converted into D-glucuronic acid, with the by-product of water. In order to reduce the cost, the multi-enzyme cascade reaction has been developed in recent years. Compared to enzyme catalysis, whole cell catalysis does not require enzyme separation and purification, making it more economical. The disadvantage is that the substrate needs to be transported across the membrane into the cell, which may reduce the reaction speed and catalytic efficiency of whole cell catalysis. At present, few microorganisms can be used to produce D-glucuronic acid, and there is no industrial trend. Recently, D-glucuronic acid has been produced from wild strain Rhizobium rosettiformans for the first time, while the related research is still in the exploratory stage, and the yield has not reached gram level.
Application of D-glucuronic acid
D-glucuronic acid has anti-inflammatory and antibacterial effects,which has important applications in the medical treatment of liver diseases. D-glucuronic acid and its derivatives can combine with endogenous and exogenous toxic substances such as phenol and hydroxyl and amino and alcohol groups in the liver to produce uronic acid substances, thus increasing the water solubility and promoting their excretion through renal urine or sweat. In the field of health care, D-glucuronic acid is widely used in cosmetics and skin care products as well as beverages and food, as it can reduce the concentration of cholesterol and triglyceride in the blood,thus aiding digestion (Fig. 1). At present,lipid-lowering drugs mainly play a role in the synthesis and catabolism of cholesterol and triglycerides. D-glucuronic acid can activate Peroxisome proliferator-activated receptorα (PPARα) and Peroxisome proliferator-activated receptorγ (PPARγ), reduce the synthesis of Low density lipoprotein cholesterol (VLDL-C), increase the activity of lipoprotein esterase, promote the decomposition of VLDL-C, inhibit the decomposition of high density lipoprotein cholesterol (HDL-C), and increase the synthesis of apolipoprotein Al and apolipoprotein A2, resulting in an increase in HDL-C and a decrease in TG (High triglycerides) and LDL-C. It has a good effect on patients with severe type IV or V hyperlipoproteinemia and coronary heart disease, but it is ineffective for diet control, weight loss and other treatments. In the field of industrial production,D-glucuronic acid is also a precursor of many important compounds, such as ascorbic acid, glucosamine and D-glucaric acid. [1]
Conclusion
D-glucuronic acid is widely used in the fields of medicine and health care products. It can be used as an intermediate to synthesize calcium D- glucuronate, 1,4-lactone D-glucuronic acid and L-ascorbic acid with anticancer effects, and can also be added to functional drinks as a food additive. Its advantages are constantly being explored, and have huge potential economic benefits.
References
1. Hu H, Li J, Jiang W, et al. Strategies for the biological synthesis of D-glucuronic acid and its derivatives. World J Microbiol Biotechnol. 2024;40(3):94. Published 2024 Feb 13. doi:10.1007/s11274-024-03900-8
2. BRAY HG. D-Glucuronic acid in metabolism. Adv Carbohydr Chem. 1953;8:251-275. doi:10.1016/s0096-5332(08)60102-2
You may like
Lastest Price from D-Glucuronic acid manufacturers

US $0.00/KG2025-09-22
- CAS:
- 6556-12-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10Tons

US $0.00/KG2025-04-21
- CAS:
- 6556-12-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500kgs/month