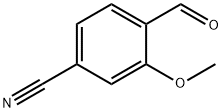
4-Formyl-3-methoxybenzonitrile: Properties and Role in Fenaridone Synthesis
The molecular structure of 4-formyl-3-methoxybenzonitrile contains three key functional groups: aldehyde (-CHO), methoxy (-OCH₃) and cyano (-CN), with the molecular formula C₉H₇NO₂, molecular weight of 161.16, and CAS Registry Number 21962-45-8. On the benzene ring skeleton, the aldehyde group is located in the meso position, the methoxy group is located in the neighboring position, and the cyano group is located in the para position, and this substitution pattern forms a stable conjugation system, which not only gives the molecule a unique electronic effect, but also provides a structural basis for its participation in a variety of organic reactions. The physical and chemical properties of 4-formyl-3-methoxybenzonitrile are closely related to its molecular structure. It is a light yellow to brown crystalline powder or solid at room temperature, with a melting point of 109-111°C, a boiling point of 318.2°C (760 mmHg), and a flash point of 138.3°C. This high melting and boiling point gives 4-formyl-3-methoxybenzonitrile good stability under conventional reaction conditions. In terms of solubility, it is readily soluble in polar organic solvents such as ethanol, dichloromethane, N,N - dimethylformamide (DMF), etc., and slightly soluble in water, which makes it easy to be operated in liquid-liquid extraction and other steps of separation and purification. In terms of intermolecular interactions, the electron-donating effect of methoxy and the strong electron-absorbing effect of 4-formyl-3-methoxybenzonitrile form a synergistic effect, which makes the aldehyde carbonyl carbon more positively charged and more susceptible to attack by nucleophilic reagents, a characteristic that makes it an excellent substrate for condensation reactions, cyclization reactions and other important organic transformations. At the same time, the presence of conjugated system makes it have a characteristic absorption in the ultraviolet visible spectral region, which provides a spectroscopic basis for its qualitative and quantitative analysis.

4-formyl-3-methoxybenzonitrile as a key intermediate in the synthesis of fenarimone
4-formyl-3-methoxybenzonitrile is a core intermediate in the synthesis of fenelidone, a hydrocorticosteroid receptor antagonist for the treatment of diabetic nephropathy, and its quality and reactivity directly determine the efficiency of fenelidone synthesis and the quality of the final product. In the improved synthetic route of fenaridone developed by Mao Yuanhu et al, this intermediate was used as a key building block in the core cyclization reaction. In the experiment, the 98% pure product provided by Shanghai Aladdin Biochemical Technology Co., Ltd. was used in the amount of 6.4 g (40 mmol), which underwent Knoevenagel condensation with 2-cyanoethyl acetoacetate (6, 8.4 g, 54 mmol) in isopropanol system under the catalytic effect of piperidine acetate (580 mg), followed by the condensation with 4-amino-5-methyl-2-hydroxypyridine through the reaction with 4-aminomethylpyridine, which was catalyzed by the reaction with 4-aminomethylpyridine and 2-methylpyridine. Subsequently, the reaction with 4-amino-5-methyl-2-hydroxypyridine was followed by Hantzsch cyclization to produce 4-(4-cyano-2-methoxyphenyl)-5-methyl-1,4-dihydro-1,6-naphthyridine-3-carboxylic acid-2-cyanoethyl ester, which had a stable yield and yielded a light yellow solid, which was the basis of the subsequent conversion. 4-Cyano-2-methoxybenzaldehyde plays a crucial role in the next process.
In the subsequent process, intermediate 9 and the original triethyl acetate O - alkylation reaction catalyzed by sulfuric acid to produce 10, and then hydrolyzed by sodium hydroxide to obtain carboxylic acid derivatives in DMF solvent, DIPEA as the acid-binding agent, PyBOP as a condensing agent, and ammonium chloride ammonia to obtain 4 - (4 - cyano - 2 - methoxyphenyl)-5 - ethoxy - 2,8 - dimethyl - 1,4 - dihydrogen - 1,6 - naphthyl - 1,6 - naphthyl - 1,6 - naphthyl - 1,6 - naphthyl - 1,8 - dimethyl - 1,8 - ethyl - 1,9 - ethyl - 2 - cyano - 3 - carboxylic acid dihydro-1,6-naphthyridine-3-carboxamide, which was ultimately split on a chiral column to give fenaridone. The introduction of 4-formyl-3-methoxybenzonitrile resulted in an overall yield of 30.8% of fenaridone (based on the starting material 3,5,5-trimethyl-4,6-dioxohex-2-en-1-one), with an end product purity of 99.7% and an ee value of 99.4%. In the improved process, the reaction conditions based on this intermediate are milder, the operation is simplified, the highly toxic reagents are avoided, the production cost is reduced, and the key technical support is provided for the industrialized production of fenarimone.
References
[1]Mao, Y., Cheng, F., He, Q., Liu, B., & Tang, L. (2022). Synthesis of Finerenone. Chinese Journal of Pharmaceuticals, 53(9), 1270–1273. https://doi.org/10.16522/j.cnki.cjph.2022.09.005
Lastest Price from 4-formyl-3-methoxybenzonitrile manufacturers
US $0.00-0.00/kg2025-08-26
- CAS:
- 21962-45-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $0.00-0.00/kg2025-04-21
- CAS:
- 21962-45-8
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 8000kg