Creatine Phosphate Disodium Salt: From Bioenergetic Buffer to Biomedical Applications
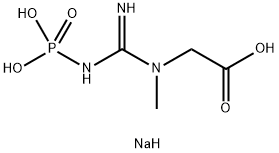
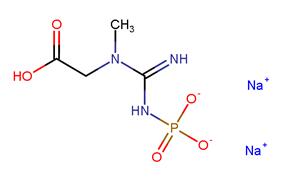
Creatine phosphate disodium salt (also known as Phosphocreatine disodium salt) is a phosphorylated creatine molecule that may serve as a quickly accessible reserve of high-energy phosphates in skeletal muscle, myocardium as well as the brain. Phosphocreatine can offer a phosphate group to ADP for ATP synthesis, or conversely, excess ATP can be used to convert creatine to creatine phosphate via creatine kinase. Thus, Creatine phosphate disodium salt could be used as a substrate for the determination of creatine kinase, and is used to regenerate ATP during skeletal muscle contraction.

Graphene oxide and Creatine phosphate disodium salt dual template
Hydroxyapatite [Ca5(PO4)3(OH), HA] is the major inorganic component of natural bones and the most stable form of phosphate salts under neutral or alkaline conditions. As the nano-size effect and surface morphology characteristics, HA nanoparticles with a high specific surface area and unique properties have wide applications in orthopedic or bone substitute which are made by biomimetic synthesis. Compared with traditional phosphate salts, the use of Creatine phosphate disodium salt as the organic phosphorus source has many advantages. Because there are no phosphate ions in the pre-reaction solution, the fast nucleation and disordered growth of calcium phosphates can be avoided. Furthermore, Creatine phosphate disodium salt can decompose to release PO43 − ions in certain conditions such as heating in aqueous solution. Under the hydrothermal process, the hydrolys can be adjusted to control the hydrolysis rate of biomolecules which determines the morphology, size, and structure of the calcium phosphate products. Herein, a facile hydrothermal method is used to prepare GO/HA hybrids constructed by CPDS/GO composite template: CPDS as a soft template and GO as a hard template. Also, CPDS serves as a phosphate source to induce the mineralization of calcium phosphate, hydroxyapatite nanoparticles.[1]
The PO43 − ions can be produced by the hydrolysis of CPDS under hydrothermal conditions, so Creatine phosphate disodium salt can be used as a biocompatible phosphate source for the synthesis of calcium phosphate salts materials. In the process of crystal-growth, there are different levels of adsorption and electrostatic attraction among CPDS, GO and Ca2 +. Owing to the adsorption of Creatine phosphate disodium salt molecules onto the surface of HA nanocrystals through electrostatic interactions and the high surface energy of the nanocrystals, the newly formed HA nanocrystals organize into a certain orientation to decrease their surface energy in aqueous solution under a hydrothermal environment. Because calcium ions are attracted by phosphate groups of CPDS and oxygen functional groups of GO. Short heating is beneficial to the interaction between GO and CPDS. While long time heating will accelerate the hydrolysis of CPDS. So the above interaction will be destroyed. Under the hydrothermal conditions, the Creatine phosphate disodium salt molecules are hydrolyzed and phosphate ions are released. HA is gradual formed in the outside of the micelle-like the complex in the alkaline condition. With the continuous hydrolysis of CPDS molecules inside the complex, the HA particles continue to accumulate, and the urchin-like spheres are formed by self-assembly.
Creatine phosphate disodium salt protects against Dox-induced cardiotoxicity
As programmed cell death, apoptosis plays a crucial role in development and in some physiological processes. The mechanism of apoptosis is complied, endoplasmic reticulum stress (ERS) as a new signal transduction pathway is researched. ERS is a condition that is accelerated by the accumulation of unfolded or mis-folded protein after a disturbance in the ER quality control system because of kinds of pathological and physiological occurrences. As a high energy compound, creatine phosphate disodium salt (CP) is more five times than ATP. Nakae et al. have proofed that it inhibited the permeability of cell membrane, changed pore of myocardial to protect the mitochondria, and even reduced apoptosis. Clinically, creatine phosphate disodium salt is used as a cardiovascular protective agent for heart diseases. In the present study, the effects of creatine phosphate disodium salt on doxorubicin-induced cardiomyocytes injury were explored; then, the potential mechanism involve in this effect was analyzed. It was proposed that doxorubicin caused ERS-induced apoptosis in cardiomyocytes injury and CP reduced ERS-induced apoptosis by calu.[2]
In summary, Creatine phosphate disodium salt protected NRC by inhibiting ERS was found, which was associated with calumenin protein. Its mechanisms may underlie and decrease the mRNA of GRP78 and GRP94 and the expression of CHOP protein. It was provided evidence of the novel evidence of CP protected against Dox-induced cardiomyocytes injury through calumenin.
References
[1]Yao, Chengli et al. “Graphene oxide and creatine phosphate disodium dual template-directed synthesis of GO/hydroxyapatite and its application in drug delivery.” Materials science & engineering. C, Materials for biological applications vol. 73 (2017): 709-715. doi:10.1016/j.msec.2016.11.083
[2]Wang, Yu et al. “Creatine phosphate disodium salt protects against Dox-induced cardiotoxicity by increasing calumenin.” Medical molecular morphology vol. 51,2 (2018): 96-101. doi:10.1007/s00795-017-0176-5
You may like
Related articles And Qustion
Lastest Price from Creatine phosphate disodium salt manufacturers

US $0.00/Kg/Bag2025-04-21
- CAS:
- 922-32-7
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 100kg

US $10.00/KG2025-04-21
- CAS:
- 922-32-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt