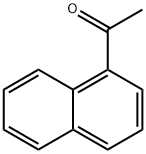
What is 1'-Acetonaphthone?
1'-Acetonaphthones are used as ingredients of fragrances. Their derivatives are used as anti-tubercular agents. The end applications include soap, detergent, beauty care product, household product. It is primarily used an intermediate in the manufacture of pharmaceutical and agrochemical products[1,2].
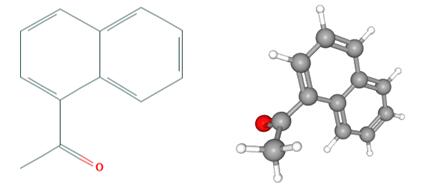
Fig 1. Chemical structure formula and three-dimensional structure of 1'-Acetonaphthone
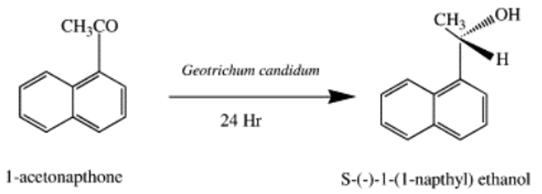
1-Acetonaphthone was incubated with the resting cells of Geotrichum candidum, Candida parapsilosis, Yarrowia lipolytica and Rhizopus arrhizus at 28 ◦C for its transformation. The first two microorganisms have shown excellent results leading to the product S(−)-1-(1-napthyl)ethanol in high yield with selectivity above 99 and 98% in the case of G. candidum and C. parapsilosis, respectively. The pH of the medium, substrate concentration and time of incubation were optimised for the maximum conversion. Adsorbent resin (XAD-7) was used for controlling cell toxicity, substrate and product inhibition. The optimum ratio of substrate: resin was found to be 1:2 for maximum yield with excellent selectivity. Subsequently, a method for the preparative bioreduction (2 g) of acetonapthone was developed by adsorbing the substrate on the resin[3].
The reduction of 1-acetonapthone was carried out with the resting cells of the above four microorganisms at 28℃ (200 rpm); however, it has been found that only two microorganisms, i.e. G. candidum and C. parapsilosis have shown a substantial reduction at pH 7 (Fig. 1) (data for other two organisms are not shown here). Hence all the subsequent experiments were carried out with G. candidum and C. parapsilosis. From Fig. 1 it is evident that maximum conversion of 1-acetonapthone to S(−)-1-(1′-napthyl) ethanol took place at 24 h with both the microorganisms, however, conversion was much higher (84%) with G. candidum than with C. parapsilosis (43%). G. candidum turned out to be the better one in terms of alcohol yield. The overall enantioselectivity of the product was, however, excellent in both the cases (Fig. 2) and only marginally higher in the case of G. candidum. Subsequently, the experiments were carried out with G. candidum only. The experiments with the resting cells of G. candidum were further carried out at different pHs ranging from 6 to 7.5 and pH 7 was found to be optimum for conversion. In the acidic region the conversion was approximately 70%[4].
References
[1] Huwaida M. E. Hassaneen. Chemistry of the Enaminone of 1-Acetylnaphthalene under Microwave Irradiation Using Chitosan as a Green Catalyst.Molecules.,2011,16(1), 609-623.
[2] Amrita Roy, et al. Hydrogen Peroxide/Boric Acid: An Efficient System for Oxidation of Aromatic Aldehydes and Ketones to Phenols.Synth. Commun.,1999,29(21), 3781-3791.
[3] A. Roy, M.S. Bhattacharyya, L. Ravi Kumar. Microbial reduction of 1-acetonapthone: A highly efficient process for multigram synthesis of S(-)-1-(1′-napthyl) ethanol[J]. Enzyme & Microbial Technology, 2003, 33(5):576-580.
[4] https://pubchem.ncbi.nlm.nih.gov/compound/13663
[5] http://www.chemspider.com/Chemical-Structure.13074.html?rid=3f828709-dcb5-4a97-86ce-f550830ce777&page_num=0
Related articles And Qustion
See also
Lastest Price from 1'-Acetonaphthone manufacturers

US $1.00/g2025-08-20
- CAS:
- 941-98-0
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $45.00/kg2025-04-21
- CAS:
- 941-98-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 tons