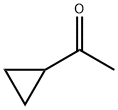
What is Cyclopropyl methyl ketone?
Cyclopropyl methyl ketone is a molecule with two large-amplitude motions: a CH3 group rotation and an acetyl C(O)CH3 group rotation, which can be coupled.Cyclopropyl methyl ketone was used in the preparation of homoallylic alcohols. Cyclopropyl methyl ketone is used as an intermediate for the manufacturing pharmaceuticals, agrochemicals and other organic compounds. Store away from strong oxidizing agents. Keep container tightly closed.
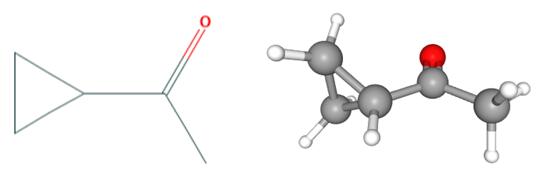
Fig 1. Chemical structure formula and three-dimensional structure of Cyclopropyl methyl ketone
Cyclopropyl methyl ketone was used in the preparation of homoallylic alcohols. Acetylcyclopropane is used as an intermediate for the manufacturing pharmaceuticals, agrochemicals and other organic compounds[1,2].
Cyclopropyl Methyl Ketone is a chemical reagents used in the synthesis of PDE4 inhibitors. Also used in the synthesis of α-trifluoromethyl-amines.
Cyclopropyl methyl ketone is a kind of important organic raw materials and intermediates. In medicine, it is mainly used for synthesizing anti-HIV drugs EFAVIRENZ and Yierleimin; In terms of pesticides, it is mainly used for fungicides, such as Cyprodinil and Cyproconazole. In herbicide, it is used as the intermediate for Isoxaflutole.
Cyclopropyl methyl ketone was first prepared in 1887 by thermal decomposition of acetylcyclopropaneearboxyltc acid. However, this synthesis method and also the oxidation of eyelopropylalkenes have not found wide application due to the difficulty of preparing the starting materials. The synthesis of cyclopropyl methyl ketone from cyclopropyl cyanide and CHsMgBr Is not promising due to the difficulty of preparing eyclopropyl cyanide and the low yield (40%) of the desired product. The preparation of cyclopropyl methyl ketone by distillation of trlmethyl-4-oxopentylammonlum salts with KOH is of purely theoretical interest. 869 g of 1-ehloro-4-pentanone was added over a period of 2 hours with cooling in ice to 790 g of powdered, chemically pure KOH. Cooling was then stopped and the reaction mixture left, overnight. On the following day the mixture was heated to 60-75ºwith stirring for 8 hours; then 600 ml of warm water was added to it and stirring continued at 60 ~ for 2 hours. When the mixture had cooled, the upper layer was separated and dried with KOH (~50 g) and water added to the lower layer until all the solid precipitate had dissolved (~4 liters).The aqueous solution was their extracted three times with ether and the ether extracts (0.9 liter) dried with KOH (~50 g). After removal of the ether, the residue and the upper layer were distilled on a column. We obtained 578 g of Cyclopropyl methyl ketone[3].
References
[1]L. S. Bartell.; J. P. Guillory.; Andrea T. Parks. Electron Diffraction Study of the Structure and Conformational Behavior of Cyclopropyl Methyl Ketone and Cyclopropanecarboxylic Acid Chloride1a. J. Phys. Chem. 1965, 69, (9), 3043-3048.
[2]William G. Dauben.; Richard E. Wolf. Transition-state conformations in the reductive opening of cyclopropyl methyl ketones. J. Org. Chem. 1970, 35, (7), 2361-2367.
[3]Wolfgang K. Process for preparing high-purity cyclopropyl methyl ketone[J]. 2000.
[4]https://pubchem.ncbi.nlm.nih.gov/compound/13004
[5]http://www.chemspider.com/Chemical-Structure.12463.html?rid=498ea016-cd06-46c9-94d6-d8961423821d
You may like
Related articles And Qustion
Lastest Price from Cyclopropyl methyl ketone manufacturers

US $8.90/KG2025-04-21
- CAS:
- 765-43-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $9.10/KG2024-10-11
- CAS:
- 765-43-5
- Min. Order:
- 1KG
- Purity:
- 99.%
- Supply Ability:
- 10000