4-Amino-3-hydroxybenzoic acid:a fundamental building block
Introduction
4-Amino-3-hydroxybenzoic acid is a chemical compound with the molecular formula C7H7NO4. It is also known by other names, such as 3-amino-4-hydroxybenzoic acid or gamma-amino-beta-hydroxybenzoic acid. It is a derivative of benzoic acid, and the arrangement of the amino and hydroxy groups on the benzene ring gives it specific properties and potential applications in various fields, including organic synthesis and pharmaceuticals.
Application
In the realm of pharmaceuticals, this compound serves as a pivotal intermediate in drug synthesis. Its incorporation into the molecular structure of pharmaceutical compounds during synthesis contributes to the development of diverse drugs. Additionally, 4-Amino-3-hydroxybenzoic acid finds utility in biochemical studies, playing a role in the synthesis of compounds with biological activity. This application supports advancements in drug discovery and understanding biological processes.In the context of research and development, the compound is employed for chemical research due to its distinctive properties and reactivity. Scientists explore its characteristics, potentially uncovering novel applications and enhancing chemical processes. This aspect underscores its role as a subject of interest in ongoing research endeavors.Furthermore, derivatives of 4-Amino-3-hydroxybenzoic acid contribute to the production of dyes and pigments as essential dye intermediates. The compound's structure plays a crucial role in determining the coloration and properties of final dye and pigment products. This application highlights its relevance in the manufacturing processes of colorants used in various industries. Bordetella sp. strain 10d metabolizes 4-amino-3-hydroxybenzoic acid via 2-hydroxymuconic 6-semialdehyde. Cell extracts from 4-amino-3-hydroxybenzoate-grown cells showed high NAD(+)-dependent 2-hydroxymuconic 6-semialdehyde dehydrogenase, 4-oxalocrotonate tautomerase, 4-oxalocrotonate decarboxylase, and 2-oxopent-4-enoate hydratase activities, but no 2-hydroxymuconic 6-semialdehyde hydrolase activity. These enzymes involved in 4-amino-3-hydroxybenzoate metabolism were purified and characterized. When 2-hydroxymuconic 6-semialdehyde was used as substrate in a reaction mixture containing NAD(+) and cell extracts from 4-amino-3-hydroxybenzoate-grown cells, 4-oxalocrotonic acid, 2-oxopent-4-enoic acid, and 4-hydroxy-2-oxovaleric acid were identified as intermediates, and pyruvic acid was identified as the final product. A complete pathway for the metabolism of 4-amino-3-hydroxybenzoic acid in strain 10d is proposed. Strain 10d metabolized 2-hydroxymuconic 6-semialdehyde derived from 4-amino-3-hydroxybenzoic acid via a dehydrogenative route, not via a hydrolytic route2. This proposed metabolic pathway differs considerably from the modified meta-cleavage pathway of 2-aminophenol and those previously reported for methyl- and chloro-derivatives. In summary, 4-Amino-3-hydroxybenzoic acid emerges as a versatile compound with applications ranging from pharmaceutical synthesis and biochemical studies to organic synthesis and contributions to the dye and pigment industry. Its unique properties position it as a valuable component in diverse chemical processes and ongoing research initiatives.
Synthesis
In the field of organic synthesis, the compound acts as a fundamental building block. Its unique structure enables chemists to construct more complex molecules for a myriad of applications. This versatility positions 4-Amino-3-hydroxybenzoic acid as a valuable asset in the creation of various compounds through organic synthesis methodologies. Microbial production of aromatic chemicals is an attractive method for obtaining high-performance materials from biomass resources. A non-proteinogenic amino acid, 4-amino-3-hydroxybenzoic acid (4-Amino-3-hydroxybenzoic acid), is expected to be a precursor of highly functional polybenzoxazole polymers; however, methods for its microbial production have not been reported. In this study, we attempted to produce 4-Amino-3-hydroxybenzoic acid from glucose by introducing 3-hydroxylation of 4-aminobenzoic acid (4-ABA) into the metabolic pathway of an industrially relevant bacterium, Corynebacterium glutamicum.
Six different 4-hydroxybenzoate 3-hydroxylases (PHBHs) were heterologously expressed in C. glutamicum strains, which were then screened for the production of 4-Amino-3-hydroxybenzoic acid by culturing with glucose as a carbon source. The highest concentration of 4-Amino-3-hydroxybenzoic acid was detected in the strain expressing PHBH from Caulobacter vibrioides (CvPHBH). A combination of site-directed mutagenesis in the active site and random mutagenesis via laccase-mediated colorimetric assay allowed us to obtain CvPHBH mutants that enhanced 4-Amino-3-hydroxybenzoic acid productivity under deep-well plate culture conditions. The recombinant C. glutamicum strain expressing CvPHBHM106A/T294Sand having an enhanced 4-ABA biosynthetic pathway produced 13.5 g/L (88 mM) 4-Amino-3-hydroxybenzoic acid and 0.059 g/L (0.43 mM) precursor 4-ABA in fed-batch culture using a nutrient-rich medium. The culture of this strain in the chemically defined CGXII medium yielded 9.8 C-mol% of 4-Amino-3-hydroxybenzoic acid from glucose, corresponding to 12.8% of the theoretical maximum yield (76.8 C-mol%) calculated using a genome-scale metabolic model of C. glutamicum1.
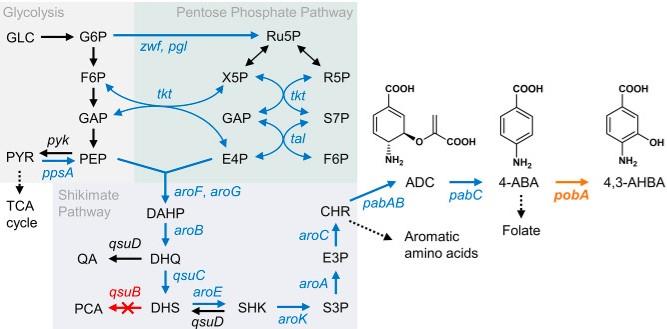
Figure 1. Schematic representation of the artificial pathway for the production of 4,3-AHBA from glucose in Corynebacterium glutamicum. Italic text indicates relevant genes in the pathway.
Safety
If 4-Amino-3-hydroxybenzoic acid is used commercially or industrially, an MSDS or its equivalent would provide information on its properties, handling, storage, and safety precautions. toxicology studies conducted on the compound can provide insights into its potential adverse effects on living organisms. Scientific studies and toxicology data may offer detailed information on the potential toxic effects of the compound. Research papers, articles, and toxicological databases could provide insights into its impact on living organisms, including humans. Regulatory Agencies: National and international regulatory agencies, such as the U.S. Environmental Protection Agency (EPA), the European Chemicals Agency (ECHA), or similar organizations in other regions, may conduct assessments and provide safety guidelines for certain chemicals. Their reports and guidelines can offer valuable information on the safety of 4-Amino-3-hydroxybenzoic acid.
Reference
1. Nonaka K, Osamura T, Takahashi F. A 4-hydroxybenzoate 3-hydroxylase mutant enables 4-amino-3-hydroxybenzoic acid production from glucose in Corynebacterium glutamicum. Microb Cell Fact. 2023 Aug 29;22(1):168.
2. Orii C, Takenaka S, Murakami S, Aoki K. Metabolism of 4-amino-3-hydroxybenzoic acid by Bordetella sp. strain 10d: A different modified meta-cleavage pathway for 2-aminophenols. Biosci Biotechnol Biochem. 2006 Nov;70(11):2653-61.
You may like
Related articles And Qustion
Lastest Price from 4-Amino-3-hydroxybenzoic acid manufacturers
US $0.00-0.00/kg2025-08-26
- CAS:
- 2374-03-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $0.00-0.00/KG2025-05-07
- CAS:
- 2374-03-0
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS