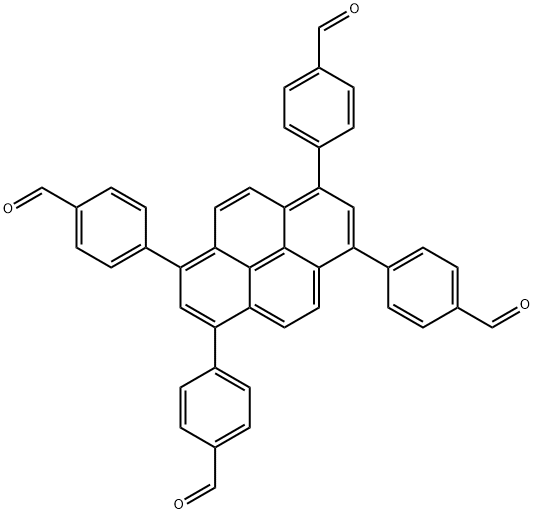
what is 4,4',4'',4'''-(pyrene-1,3,6,8-tetrayl)tetrabenzaldehyde?
4,4',4'',4'''-(pyrene-1,3,6,8-tetrayl)tetrabenzaldehyde is representative functional π-systems, which have attracted significant attention in the fields of chemistry, materials science, and nanotechnology because it plays vital roles in numerous applications. 4,4',4'',4'''-(pyrene-1,3,6,8-tetrayl)tetrabenzaldehyde- is typical π-macrocycles with extended π-conjugation that enables coordination with almost all transition metals to form metalloporphyrins and provides diverse unique functions, including optical, photochemical, catalytic, and redox properties. These features render it invaluable in science and technology is an important organic intermediate (building block) to synthetize substituted products.
4,4',4'',4'''-(pyrene-1,3,6,8-tetrayl)tetrabenzaldehyde- is also an important organic intermediate (building block) to synthetize substituted Porphyrin products.
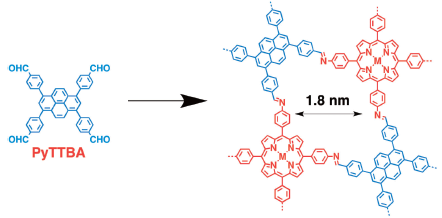
Chen et al. reported [1] the designed synthesis of porphyrin-based two-dimensional covalent organic frameworks with highly ordered structures. The porphyrin units were covalently integrated into the 2D polymer sheets that were further stacked to constitute layered frameworks. Consequently, the porphyrin units are located at the vertices of extended tetragonal polygons, giving rise to periodically ordered columnar porphyrin π-arrays. Tuning the size and geometry of the edge units allows the porphyrin frameworks to be tailored to possess tetragonal or rhombic polygons with pore sizes ranging from micropores to mesopores. The frameworks are highly porous, exhibit extended π-conjugation, and show improved light absorption capability. The extended porphyrin frameworks will create new opportunities in the chemistry of porphyrins and related macrocycles by offering unique structures that can be elaborately designed and can be synthesized in a straightforward manner via polycondensation.
Waller et al. reported [2] the utility of a method based on 1,3,6,8-tetrakis(4-formylphenyl)-pyrene substitution followed by oxidative cyclization in producing new COFs of new linkages. This method is promising in advancing COF chemistry from the usual reversible linkages to more desirable and difficult to make linkages of limited reversibility. The high crystallinity and porosity of these materials are a testament to the value of this method, which translates the quality of the imine precursor to more irreversible chemistries.
Li et al. reported [3] its application on the light-emitting covalent organic frameworks: fluorescence improving via pinpoint surgery and selective switch-on sensing of anions. Deprotonation of the N−H bond to form an anionic nitrogen species in the hydrazine linkage can eliminate the nitrogen-related fluorescence quenching pathway. The resulting COF enhances the fluorescence in a linear proportion to the progress ofdeprotonation, achieving a 3.8-fold improved emission. This pinpoint N−H cleavage on the pore walls can be driven only by the fluoride anion while other halogen anions, including chloride, bromide, and iodide, remain inactive, enabling the selective fluorescence switch-on sensing of the fluoride anion at a ppb level.
References
1. Chen X, Gao J, Jiang D. Designed Synthesis of Porphyrin-based Two-dimensional Covalent Organic Frameworks with Highly Ordered Structures[J]. Chem. Lett. 2015, 44:1257–1259
2. Waller P et al. Conversion of Imine to Oxazole and Thiazole Linkages in Covalent Organic Frameworks[J]. J. Am. Chem. Soc. 2018, 140:9099−9103
3.Li Z et al. Light-Emitting Covalent Organic Frameworks: Fluorescence Improving via Pinpoint Surgery and Selective Switch-On Sensing of Anions[J]. J. Am. Chem. Soc. 2018, 140:12374−12377
See also
Lastest Price from 4,4',4'',4'''-(pyrene-1,3,6,8-tetrayl)tetrabenzaldehyde manufacturers
US $10.00-50.00/KG2025-06-18
- CAS:
- 1415238-25-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $10.00-110.00/kg2024-08-27
- CAS:
- 1415238-25-3
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20