ChemicalBook > Articles Catagory List >Organic-Chemistry >what-is-1-ethyl-3-methyl-1h-pyrazole-4-carbaldehyde-
What is 1-Ethyl-3-methyl-1H-pyrazole-4-carbaldehyde?
Feb 17,2020
1-Ethyl-3-methyl-1H-pyrazole-4-carbaldehyde (C7H10N2O, CAS registry No. 676348-38-2) is a commercially available aldehyde. Its flash point is 100.3°C. It can cause skin irritation and serious eye irritation. It should be stored in cool place and should be kept container tightly closed in a dry and well-ventilated place.
1-Ethyl-3-methyl-1H-pyrazole-4-carbaldehyde can be used as substrate for the synthesis of many important compounds. For example, it can be used as the substrate to synthesize pyrazole derivative via Baeyer-Villiger oxidation[1]. The obtained pyrazole derivative can be used to synthesize a series of orexin receptor dual antagonists.
It can also be used as the substrate to synthesize N-tert-butanesulfinyl imines with (R)-tert-butanesulfinamide in the presence of titanium tetraisopropoxide at 50 oC[2]. Then these obtained N-tert-butanesulfinyl imines were used to synthesize diastereomeric homoallylsulfinamides by the In and Zn-mediated allylation depending on the conditions and additives (up to 99.4% de). These diastereomeric homoallylsulfinamides were then converted into the corresponding pyrazol-4-yl-derived N-Boc-homoallylamines via consecutive treatment with HCl and Boc2O. The latter were then subjected to a sequence of reactions: cyclobromocarbamation with NBS and enolate-isocyanate rearrangement with tBuOK to give novel enantiomerically pure (S)-6-(pyrazol-4-yl)-piperidine-2,4-diones, which might be helpful in the design and synthesis of new pharmaceuticals, because both of pyrazole and piperidine-2,4-dione fragments are active pharmacophores.
It also can be used for the synthesis of 3-amino-isoquinolines, such as (N-(6,7-dimethoxy-1-methyl-isoquinolin-3-yl)-4-{[(1-ethyl-4-methyl-1H-pyrazol-3-yl)methyl]amino}benzamide, which exhibited the significant effect against different tumor cell lines while showing the high activity toward human colorectal cancer HCT-116 cells (IC50 = 18 μM) and human breast cancer T-47D cells (GI50 = 1.9 μM). And the target protein of these compounds are kinases and phosphodiesterases (PDE)[3].
Another example is that 1-ethyl-3-methyl-1H-pyrazole-4-carbaldehyde can be used as material for the the formation of 5,8-Dihydropyrido[2,3-d]pyrimidine skeleton in aqueous media at facile energy-efficient and environmental-friendly conditions using green microwave-assisted multicomponent reaction[4].
It can also participate in the aminoallylation reaction with ammonia and aliphatic amines as stoichiometric allylating agents to synthesize adducts of triallylborane in methanol[5].
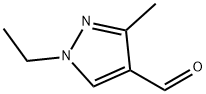
Fig 1. Chemical structure formula of 1-ethyl-3-methyl-1H-pyrazole-4-carbaldehyde
References
[1].Yoshida, Y.; Naoe, Y.; Terauchi, T.; Ozaki, F.; Doko, T.; Takemura, A.; Tanaka, T.; Sorimachi, K.; Beuckmann, C. T.; Suzuki, M.; Ueno, T.; Ozaki, S.; Yonaga, M., Discovery of (1R,2S)-2-{[(2,4-Dimethylpyrimidin-5-yl)oxy]methyl}-2-(3-fluorophenyl)-N-(5-fluor opyridin-2-yl)cyclopropanecarboxamide (E2006): A Potent and Efficacious Oral Orexin Receptor Antagonist. Journal of medicinal chemistry 2015, 58 (11), 4648-64.
[2].Kuznetsov, N. Y.; Khrustalev, V. N.; Strelkova, T. V.; Bubnov, Y. N., Diastereoselective In and Zn-mediated allylation of pyrazol-4-yl derived (R)-tert-butanesulfinyl imines: synthesis of enantiomerically pure 6-(pyrazol-4-yl)-piperidin-2,4-diones. Tetrahedron: Asymmetry 2014, 25 (8), 667-676.
[3].Lapa, G. B.; Tsunoda, T.; Shirasawa, S.; Baryshnikova, M. A.; Evseev, G. G.; Afanasyeva, D. A.; Chigorina, E. A., Synthesis of New Congeners of 1-methyl-3-aminoisoquinolines, Evaluation of Their Cytotoxic Activity, In Silico and In Vitro Study of Their Molecular Targets as PDE4B. Chemical biology & drug design 2016, 87 (4), 575-82.
[4].Saraev, V. E.; Zviagin, I. M.; Melik-Oganjanyan, R. G.; Sen'ko, Y. V.; Desenko, S. M.; Chebanov, V. A., Green Microwave-assisted Multicomponent Route to the Formation of 5,8-Dihydropyrido[2,3-d]pyrimidine Skeleton in Aqueous Media. Journal of Heterocyclic Chemistry 2017, 54 (1), 318-324.
[5].Kuznetsov, N. Y.; Tikhov, R. M.; Strelkova, T. V.; Bubnov, Y. N., Adducts of Triallylborane with Ammonia and Aliphatic Amines as Stoichiometric Allylating Agents for Aminoallylation Reaction of Carbonyl Compounds. Org Lett 2018, 20 (12), 3549-3552.