ChemicalBook > Articles Catagory List >Pharmaceutical-intermediates >what-is-1-4-piperazinediethanesulfonic-acid-pipes-
What is 1,4-Piperazinediethanesulfonic acid (PIPES)?
Feb 14,2020
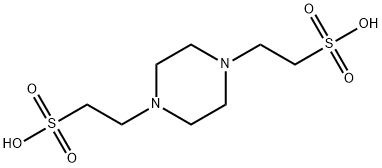
Pipes (PIPES0215; PIPERAZINE-N, N'-BIS (2-ETHANESULFONIC ACID) PIPERAZINE -N,N'-BIS(2-ETHANESULFONIC ACID) PIPERAZINE-N, N'-BIS (2-ETHANESULFONIC ACID) PIPERAZINE-N, N'-BIS (2-ETHANESULFONIC ACID); 1,4-Piperazinediethanesulfonic acid (PIPES), 99.5%; PIPES, Free Acid, ULTROL; PIPES (PIPERAZINE-1,4-BIS (2-ETHANESULFONIC ACID)); PIPES ULTRAPURE; PIPERAZINE-N, N-BIS (2-ETHANESULFONICACID) (PIPES); Piperazine-N, N'-bis- (2-ethanesulphonic acid) ultra -pure >99.5%)[1] is an important chemical intermedia in all kinds of chemical fields. And 1,4-Piperazinediethanesulfonic acid, piperazine-N, N’-bis- (2-ethanesulfonic acid) (PIPES) is also one of the zwitterion buffers and the most appropriate buffer for the biological experiments using an intracellular solution since the Pk2 of Pipes is 6.8 at 20 degrees Celsius. Moreover, it is considered to be nontoxic and metabolically inert. 1,4-Piperazinediethanesulfonic acid (PIPES), a zwitterion buffer, could be titrated with KOH to measure its pH and osmolality. Both pH and osmolality changes showed three phases, and two transition points coincided with pH 5.1 and 10.2. The second phase is important in biological reactions and from the present results, the Pk1 is 3.3 while pK2 is 6.85 at 20 degrees Celsius.[2]
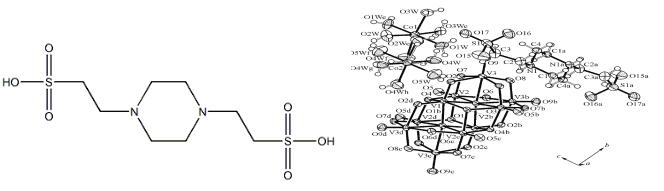
The values of the ionic strength of PIPES calculated previously differ among various investigators. Some investigators estimate the valence of PIPES to be high and others low. Junna Hatae et al.[2] have set up three hypotheses and compared the experimental values, then determined an appropriate valence of PIPES around the physiological intracellular pH. At the same time, 1,4-Piperazinediethanesulfonic acid (PIPES) is also used widely in the field of synthesis. S.-Y.Luo et al.[3] revealed that different moieties in the compound show a three-dimensional framework structure, in which {CoO6}, the decavandate cluster anions, and 1,4-piperazinediethanesulfonic acid (PIPES) interact with each other by intermolecular forces and strong hydrogen bonding. Bond valence calculations were used to calculate the valence states of the atoms. In addition, Víctor Rodríguez-Sureda et al.[4] found a procedure for measuring triacylglyceride and cholesterol content using a small amount of tissue, which of the second step. extracted lipids are emulsiWed by sonication in a buVer containing 1,4-piperazinediethanesulfonic acid (28.75mM), magnesium chloride·6H2O (57.76mM), free fatty acids–bovine serum albumin (8.76 µM), and sodium dodecyl sulfate (0.1%).
References
[1] https://www.chemicalbook.com/ProductChemicalPropertiesCB4435134_EN.htm
[2] HATAE J, FUJISHIRO N, KAWATA H. Determination of the appropriate valence of 1, 4-piperazinediethanesulfonic acid (PIPES) in physiological pH[J]. Biological and Pharmaceutical Bulletin, 1994, 17(3): 437-439.
[3] Luo S Y, Wu X L, Hu Q P, et al. Structural characterization of a new decavanadate compound with organic molecules and inorganic ions[J]. Journal of Structural Chemistry, 2012, 53(5): 915-920.
[4] Rodríguez-Sureda V, Peinado-Onsurbe J. A procedure for measuring triacylglyceride and cholesterol content using a small amount of tissue[J]. Analytical biochemistry, 2005, 343(2): 277-282.
Related articles And Qustion
See also
Lastest Price from PIPES manufacturers
PIPES

US $30.00/kg2025-04-21
- CAS:
- 5625-37-6
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 100 tons
PIPES

US $30.00-10.00/KG2025-04-15
- CAS:
- 5625-37-6
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 500000kg