What is Dibutyltin oxide?
Feb 14,2020
Dibutyltin oxide (Bu2SnO, C8H18OSn, CAS registry No. 818-08-6) is a white fine powder. Its flash point is 81-83 °C. The solubility of HNQ is 4.0 mg/L in water at 20 °C. It is flammable when exposed to flame. It can react with oxidizing materials. To fight fire, use dry chemical, fog, CO2. When heated to decomposition, it emits acrid smoke and irritating fumes.
Dibutyltin oxide can be used as the catalyst in organic reaction. For example, Bu2SnO can be used as the catalyst for the selective deacylation of esters of glycosyl units in the synthesis of various free-OH glycosides containing base- or acid-sensitive multifunctional groups under mild conditions[1]. Dibutyltin oxide can also catalyze the allyl-transfer reaction from tertiary homoallylic alcohols to aldehydes in toluene under reflux conditions[2]. Various secondary homoallylic alcohols were prepared in high yield (up to 99%). The plausible catalytic mechanism of the allyl-transfer reaction of an allyl donor to an aldehyde have been proposed. Initially, the allyl donor reacts with Bu2SnO, forming the tin alkoxide of the tertiary homoallylic alcohol. The alkoxide then undergoes ‘retro-allylation’ to provide an allylic tin compound and benzophenone. Subsequently, the allylic tin is allowed to add to the aldehyde. Finally, reaction of the resulting tin alkoxide with the allyl donor completes the catalytic cycle, yielding the secondary homoallylic alcohol (branched product) and regenerating the tin alkoxide of the allyl donor.
Dibutyltin oxide is known to react with hydroxyl groups at 1,3- or 1,4-positions of cyclic polyalcohols such as glycosides, forming five or six membered rings such as C(glyc.)-O-Sn(Bu)2-O-C(glyc.). Dibutyltin oxide can also react with graphite oxide (GO), which possesses a large amount of acidic hydroxyl groups on its layer[3]. The resulting products would contain tin atoms dispersed in GO at a molecular level. By pyrolyzing them, carbons containing nano-sized tin species will be obtained, which is promising as anodes of lithium ion battery.
Dibutyltin oxide can react with the fluorouracil compounds 5-fluorouracil-1-propanonic or 5-fluorouracil-1-acetic acid (Fu) to give the complexes [(5-Fu)-1-(CH2)nCOOSn(n-Bu)2]4O2 (I, n=2; II, n=1), which have potential anti-tumor activity: in vitro tests showed that complexes I and II exhibit high cytotoxicity against tumor cells of OVCAR-3 and PC-14[4].
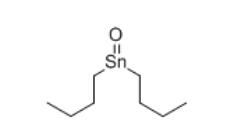
Fig 1. Chemical structure formula of dibutyltin oxide
References
[1].Liu, H. M.; Yan, X. B.; Li, W.; Huang, C. H., A mild and selective method for cleavage of O-acetyl groups with dibutyltin oxide. Carbohydrate Research 2002, 337 (19), 1763-1767.
[2].Yanagisawa, A.; Aoki, T.; Arai, T., Dibutyltin oxide catalyzed allyl-transfer reaction from tertiary homoallylic alcohols to aldehydes. Synlett 2006, (13), 2071-2074.
[3].Matsuo, Y.; Matsumoto, Y.; Fukutsuka, T.; Sugie, Y., Reaction between dibutyltin oxide and graphite oxide. Carbon 2006, 44 (14), 3134-3135.
[4].Zuo, D. S.; Jiang, T.; Guan, H. S.; Wang, K. Q.; Qi, X.; Shi, Z., Synthesis, structure and antitumor activity of dibutyltin oxide complexes with 5-fluorouracil derivatives. Crystal structure of (5-fluorouracil)-1-CH2CH2COOSn(n-Bu)(2) (4)O-2. Molecules 2001, 6 (8), 647-654.