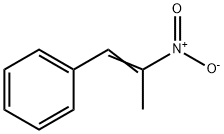
What is 1-Phenyl-2-nitropropene?
Identification
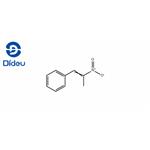
Product Name: 1-Phenyl-2-nitropropene
Synonyms: 1-(PHENYL) 2-NITROPROPENE;2-NITROPROP-1-ENYLBENZENE;2-NITRO-1-PHENYLPROPENE;BETA-METHYLNITROSTYRENE;BETA-METHYL-BETA-NITROSTYRENE;TIMTEC-BB SBB003925;TRANS-BETA-METHYL-BETA-NITROSTYRENE;trans-β-methyl-β-nitrostyrene
CAS: 705-60-2
MF: C9H9NO2
MW: 163.17
EINECS: 627-363-3
Chemical Properties
Melting point 63-65 °C (lit.)
Boiling point 263.0±9.0 °C(Predicted)
Density 1.141±0.06 g/cm3(Predicted)
Storage temp. 2-8°C
Form Crystalline Powder
Color Yellow
1-Phenyl-2-nitropropene, or simply Phenyl-2-nitropropene, or P2NP, as it is commonly referred to, is a chemical compound from the aromatic group of compounds, with the formula C9H9NO2. It is a crystalline solid of light yellow color with a distinct smell. Phenyl-2-nitropropene is used in the pharmaceutical industry to manufacture the drug Adderall, an amphetamine mixture used to treat ADHD and narcolepsy. P2NP and other similar nitrostyrenes are also employed in the clandestine manufacture of drugs of the amphetamine class, and are listed as drug precursors in many countries.
Uses
1-phenyl-2-nitropropene is used in the production of pharmaceuticals, for instance, for drug Adderall, a drug used to treat ADHD and narcolepsy.
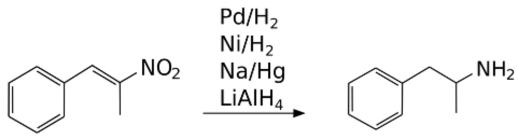
Reduction of P2NP to Amphetamine
The above diagram depicts P2NP reduction to amphetamine by using either LAH, Sodium amalgam, Raney Nickel or Palladium (Pd) catalyst, as a reducing agent. Platinum (Pt) in the form of Adams' catalyst can also be used.
Synthesis
Phenyl-2-nitropropene is a chemical compound. It can be produced by the reaction of benzaldehyde and nitroethane in the presence of a basic catalyst. It can be reduced in the presence of a catalyst to produce phenylacetone, which is a controlled precursor of methamphetamine. With lithium aluminium hydride, it can be reduced to amphetamine.
(Methylamine Catalysis)
1 mol benzaldehyde, 1.2 mol nitroethane and 15 mL diluted aqueous methylamine in 150 mL alcohol. Stirred and slightly heated for ca 4 hours. The reaction mixture is brought over into a beaker and cooled in the fridge (4°C). If precipitation doesn't commence at this point, water is poured in and the mixture put back in the fridge. The P2NP oil layer will slowly (or sometimes quickly) start forming a crystalline layer. If it doesn't, scratching the wall with e.g. a glass rod will help. If even this won't help, it means you probably fucked something up. The yields I have had using this "adaptation": 81% (i-PrOH), 79% (i-PrOH), 75% (EtOH) and 71% (EtOH) (yields calculated after crystallizing once; I store my crystals in the freezer and also recrystallize them prior to use).
(Cyclohexylamine Catalysis)
To 55 g (0.5mol) Benzaldehyde in a 500mL Flask were added 40 g (0.5mol) Nitroethane and 10mL Cyclohexylamine. All was refluxed for 6h on a water bath. The result were 2 layers. One orange layer at the bottom with phenyl-2-nitropropene and a clear layer at the top with cyclohexylamine and maybe a little bit (~1mL) of H2O. 50mL of H2O were added and then sucked off with a pipette until the phenyl-2-nitropropene crystallized (it crystallized when it came in contact with air in presence of 15mL H2O). I added 200mL 95% denaturated ethanol to the orange crystals. The color of the now needle-like crystals changed from orange to white-yellow. The crystals were filtered. Yield 65 g, 78% of theory.
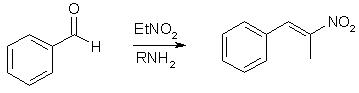
Toxicity
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 154mg/kg (154mg/kg) | Chemotherapy Vol. 11, Pg. 321, 1963. | |
| mouse | LD50 | oral | 1176mg/kg (1176mg/kg) | Chemotherapy Vol. 11, Pg. 321, 1963. | |
| rat | LD | oral | > 500mg/kg (500mg/kg) | National Academy of Sciences, National Research Council, Chemical-Biological Coordination Center, Review. Vol. 5, Pg. 32, 1953. | |
| rat | LDLo | intraperitoneal | 200mg/kg (200mg/kg) | Biochemical Pharmacology. Vol. 13, Pg. 285, 1964. |
Safety and Storage
P2NP is labeled as Harmful by the Globally Harmonized System of Classification and Labelling of Chemicals(GHS), and is a known irritant, thus breathing fumes and direct skin and eye contact must be avoided. The median lethal dose for oral exposure in rats and mice is greater than 500 mg/kg and 1176 mg/kg, respectively. Based on available data, the classification criteria are not met to classify Phenyl-2-Nitropropene as a carcinogen, however, the toxicological properties of p2np have not been thoroughly investigated.
P2NP should be stored at 2°C to 8°C, away from strong oxidizing agents. At higher temperatures P2NP is not very stable, and degrades with time.
Related articles And Qustion
See also
Lastest Price from 1-Phenyl-2-nitropropene manufacturers
US $0.00/kg2025-05-15
- CAS:
- 705-60-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS

US $6.00/kg2025-04-21
- CAS:
- 705-60-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month