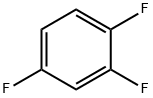
What is 1,2,4-Trifluorobenzene?
General description
1,2,4-Trifluorobenzene, with the CAS No: 367-23-7. This chemical’s molecular formula is C6H3F3 and molecular Weight is132.08g/mol. The boiling point is 88℃ at 760 mmHg. The density is 1.264 g/Cm3. The flash point is 40 °F.The appearance is colorless and transparent liquid.
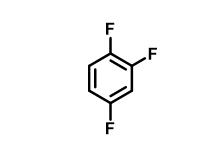

Figure 1 The structure of Methyl 6-aminonicotinate
The synthesis of 1,2,4-Trifluorobenzene
To a round bottom flask was added 6-aminonicotinic acid (2.76 g, 20 mmol) and 3 M HCl in MeOH (70 mL), the mixture was refluxed for 18 hours. After the reaction, the solvent was removed under vacuum, the residue was dissolved in ethyl acetate (200 mL) and washed with saturated Na2CO3 solution and brine, dried over Na2SO4 . The product was used in the next step without further purification; yield: 2.98 g, 98%, white solid; 1H NMR (500 MHz, CDCl3) δ 8.73 (d, J = 2.4 Hz, 1H), 8.01 (dd, J = 8.6, 2.3 Hz, 1H), 6.47 (d, J = 8.6 Hz, 1H), 5.10 (s, 2H), 3.88 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 166.3, 161.2, 151.5, 139.0, 116.5, 107.5, 51.8.

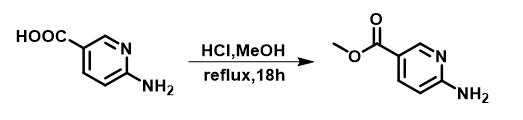
Figure 2 The synthesis of Methyl 6-aminonicotinate
Excited state lifetimes
Excited state lifetimes of Methyl 6-aminonicotinate were measured in different solvents by using λexc 293 and 311 nm, whereas the λem were the fluorescence band maximum in the respective solvents. Fluorescence intensity in each case followed a single exponential decay with χ2=1±0.1 and with good autocorrelation function. Further, it may be mentioned here that the τ are independent of λexc. Figure 3 depicts a typical fluorescence decay profile in water at pH 8. Values of radiative (kr) and non-radiative (knr) rate constants were calculated from the lifetimes (τ) and Φf using the following relations[2].

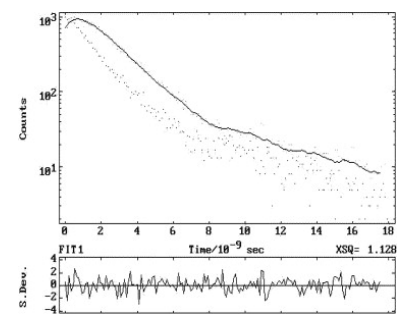
Figure 3 Fluorescence decay profile in Water (pH = 8). λex=293 nm, λem=346 nm
Effects of solvents
Absorption band maxima (λmaxab) and molecular extinction coefficients (log εmax) of methyl 6-aminonicotinate in different solvents have been compiled in
Table 3 Absorption band maximum (λmaxab, nm), molecular extinction coefficient [Math Processing Error](logεmax), fluorescence band maximum (λmaxf, nm), fluorescence quantum yield (Φf), lifetimes (τ, ns), radiative (kr, 108 s−1) and non-radiative (knr, 108 s−1) rate constants of different species and in different solvents.
Sl. No. solvent | [Math Processing Error]λmaxab(logεmax) | τ, ns | λmaxf(Φf) | kr | knr |
Cyclohexane | 265(4.317) | 0.85 | 324(0.089) | 1.05 | 10.71 |
293(3.865) | |||||
304(3.58) | |||||
Ether | 272(4.417) | 1.07 | 336(0.109) | 1.02 | 8.33 |
Dioxane | 272(4.439) | 0.97 | 339(0.097) | 1.00 | 9.31 |
Ethyl acetate | 272(4.419) | 1.11 | 338(0.099) | 0.89 | 8.12 |
Acetonitrile | 272(4.402) | 1.14 | 339(0.105) | 0.92 | 7.85 |
n-Propanol | 274(4.413) | 1.25 | 344(0.113) | 0.90 | 7.10 |
Methanol | 275(4.393) | 1.34 | 345(0.109) | 0.81 | 6.65 |
Water, pH 8 | 274(4.333) | 0.99 | 346(0.063) | 0.64 | 9.46 |
Water H_=16 | 261(4.273) | 1.03 | 444(0.016) | 0.16 | 9.55 |
293(3.903) | |||||
Water pH 2.6 | 262(4.327) | 1.11 | 364(0.098) | 0.88 | 8.12 |
301(3.913) | |||||
Water, H0=−3.0 | 260(4.32) | 1.77 | 362(0.135) | 0.76 | 4.89 |
298(3.95) | |||||
Water, H0=−10.05 | 264(4.294) | 0.5 | 359(0.031) | 0.62 | 19.38 |
296(4.028) |
Hazard description
Methyl 6-aminonicotinate is harmful to humans if swallowed, skin contact, and inhalation.
Ecological data
Methyl 6-aminonicotinate is slightly harmful to water. Do not let undiluted or large amounts of products come into contact with groundwater, water courses or sewage systems. Do not discharge materials into the surrounding environment without government permission.
Reference
[1]Yan, Gang, Golden, et al. Palladium-Catalyzed Cyclocarbonylation of Pyridinylated Vinylogous Amides and Ureas to Generate Ring-Fused Pyridopyrimidinones[J]. Organic Letters, 2018.
[2] Nayak M K , Dogra S K . Solvatochromism and prototropism in methyl 6-aminonicotinate: failure to observe amine-imine phototautomerism in solvents[J]. Journal of Molecular Structure, 2004, 702(1-3):85-94.
Lastest Price from 1,2,4-Trifluorobenzene manufacturers

US $10.00/ASSAYS2025-08-17
- CAS:
- 367-23-7
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton

US $0.00-0.00/kg2025-04-21
- CAS:
- 367-23-7
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons