Linoleic Acid: Role in Human Diet and Food Sources
General Description
Linoleic acid, a prevalent PUFA in the modern diet, is primarily found in vegetable oils, nuts, seeds, meats, eggs, and processed foods, with soybean oil contributing significantly in the US. As a crucial energy source, it is also vital in the formation of lipids and cell signaling compounds. While it can be converted to arachidonic acid, which yields bioactive compounds, excessive production is linked to chronic diseases. This article will introduce the food sources of linoleic acid and its role in human diet.

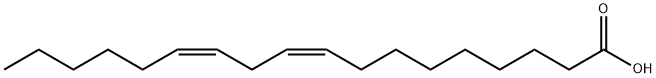
Figure 1. Linoleic acid
Food Sources
The major dietary sources of linoleic acid are vegetable oils, nuts, seeds, meats, and eggs. The consumption of linoleic acid in the US diet began to increase around 1969 and paralleled the introduction of soybean oil as the major commercial additive to many processed foods. Manufactured foods containing soybean oil as a primary ingredient will be rich in linoleic acid. Currently, soybean oil accounts for ∼45% of dietary linoleic acid in the US diet. Nevertheless, linoleic acid is also the most abundant PUFA in most foods. Although linoleic acid accounts for ∼88% of the total PUFAs in soybean oil, the levels in most commonly consumed foods exceed 70%. For example, of all the PUFAs in most meats (beef, chicken, and pork), the contribution of linoleic acid is between 70 and 85% and >80% in eggs. Although it is well recognized that most vegetable oils are linoleic acid-based (noted exception is flaxseed), even foods with very low fat content (vegetables, fruits, and grains) are predominantly rich in linoleic acid as the major PUFA. Noted exceptions are beans, in which linoleic acid comprises between 40 and 50% of the total PUFAs.1
Role in Human Diet
Linoleic acid (18:2ω6; cis, cis-9,12-octadecadienoic acid) is the most highly consumed PUFA found in the human diet. On consumption, linoleic acid has 4 primary fates. Like all fatty acids, it can be used as a source of energy. It can be esterified to form neutral and polar lipids such as phospholipids, triacylglycerols, and cholesterol esters. As part of membrane phospholipids, linoleic acid functions as a structural component to maintain a certain level of membrane fluidity of the transdermal water barrier of the epidermis. In addition, when released from membrane phospholipids, it can be enzymatically oxidized to a variety of derivatives involved in cell signaling [i.e., 13-hydroxy or 13-hydroperoxy octadecadienoic acid, 13-H(P)ODE].
As the parent compound for the family of ω6 PUFAs, linoleic acid can be elongated and desaturated to other bioactive ω6 PUFAs, such as γ-linolenic acid (18:3ω6) and arachidonic acid (20:4ω6). Subsequently, arachidonic acid can be converted to a myriad of bioactive compounds called eicosanoids, such as prostaglandins and leukotrienes. These eicosanoids are important in normal metabolic function of cells and tissues, but when persistently produced in excess, they are known to contribute to a number of chronic diseases, such as inflammation and cancer. It is this possible conversion to arachidonic acid for which linoleic acid has received the most notoriety. Although it has been hypothesized that limiting the intake of linoleic acid can reduce tissue levels of arachidonic acid, this does not seem to be the case in individuals who are consuming a typical Western diet. In tracer kinetic studies, fractional conversion of linoleic acid to arachidonic acid is believed to be between 0.3% and 0.6%, and this conversion appears to be offset by turnover.
After consumption and absorption by enterocytes lining the small intestines, linoleic acid is packaged into chylomicrons as phospholipids, triacylglycerols, or cholesterol esters and enters the general circulation (subclavian vein) via the thoracic duct. Linoleic acid is delivered to hepatic and extrahepatic tissues as chylomicrons are delipidated en route to and cleared by the liver during its transition to much smaller remnant particles. After cellular uptake, the fate of linoleic acid is determined by the needs of the tissue, i.e., incorporation into membrane phospholipids, desaturation and elongation, etc.1
Reference
1. Whelan J, Fritsche K. Linoleic acid. Adv Nutr. 2013; 4(3): 311-312.
You may like
Related articles And Qustion
See also
Lastest Price from Linoleic acid manufacturers
US $0.00-0.00/kg2025-11-21
- CAS:
- 60-33-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $135.00-65.00/kg2025-04-25
- CAS:
- 60-33-3
- Min. Order:
- 1kg
- Purity:
- 94.9%
- Supply Ability:
- 10 TONS