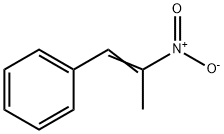
1-Phenyl-2-nitropropene: Applications in Drug Discovery and its Preparation Method
General Description
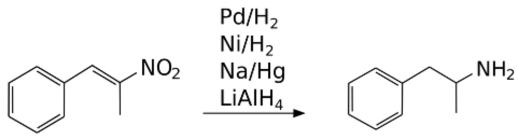
1-Phenyl-2-nitropropene is one of the important intermediate preparation of amphetamine, which iswidely used as cognitive enhancer drug of the central nervous system. It exhibits the property of thedipole moment and hyperpolarizability three times higher than the NLO property of the urea. Thereare larger charge distribution and flow of charge from electrophile to nucleophile in the molecule

Applications in Drug Discovery
1-Phenyl-2-nitropropene is used in the pharmaceutical industry to manufacture the drug Adderall, an amphetamine mixture used to treat ADHD and narcolepsy. P2NP and other similar nitrostyrenes are also employed in the clandestine manufacture of drugs of the amphetamine class, and are listed as drug precursors in many countries.
Preparation Method
To synthesize 1-Phenyl-2-nitropropene, start by mixing β-Methylstyrene, sodium nitrite (NaNO2), and 1-Butyl-3-methylimidazolium chloride ([Bmim]Cl) in a 10 mL glass vial equipped with a magnetic stirrer. The amounts used are 0.25 mmol of β-Methylstyrene and 69 mg of sodium nitrite dissolved in 0.5 mL of [Bmim]Cl. Stir the mixture at 80°C under a nitrogen atmosphere for 1 hour. After stirring, add p-methylanisole to the reaction mixture and then treat with 5% hydrochloric acid (HCl). Proceed by extracting the mixture with 0.5 mL of ethyl acetate to obtain the organic phase, which contains 1-Phenyl-2-nitropropene. This phase is then subjected to gas phase chromatography for further purification.
Purification
Following the initial extraction, cool the reaction mixture to room temperature and treat it once more with 5% HCl. Perform additional extractions with 0.5 mL of ethyl acetate twice. Concentrate the resulting solution under vacuum to remove excess solvents. Purify the crude product by chromatography on a short silica gel pad using a petroleum ether/ethyl acetate eluent. This purification step isolates 1-Phenyl-2-nitropropene, specifically in the form of (2-nitroprop-1-en-1-yl)benzene, achieving an overall yield of 52%.
References:
[1] MICHELE CASIELLO . Ionic‐Liquid Controlled Nitration of Double Bond: Highly Selective Synthesis of Nitrostyrenes and Benzonitriles[J]. European Journal of Organic Chemistry, 2020, 2020 37: 5892-6025. DOI:10.1002/ejoc.202001027.You may like
Related articles And Qustion
Lastest Price from 1-Phenyl-2-nitropropene manufacturers
US $0.00/kg2025-05-15
- CAS:
- 705-60-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS

US $6.00/kg2025-04-21
- CAS:
- 705-60-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month