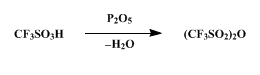
What chemical reactions can occur with Trifluoromethanesulfonic anhydride?
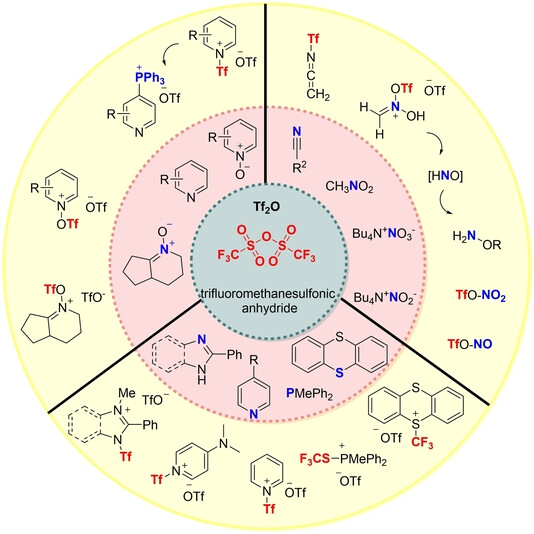
Trifluoromethanesulfonic anhydride (Tf2O) is a strong electrophilic activator that can participate in a wide range of chemical reactions and is widely used in organic synthesis to prepare novel compounds. The relevant chemical reactions of Tf2O are described below:
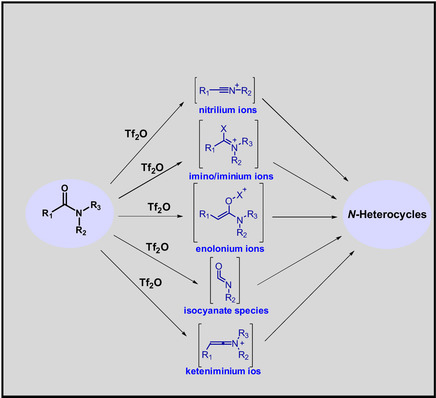
Amide activation
Tf2O is able to synthesise a variety of N-heterocycles via activated amides. This activation is associated with the formation of intermediates such as enolonium, imino/iminium, nitrilium and keteniminium triflate ions and isocyanate species which can lead to the synthesis of different types of N-heterocycles depending on the nature of the intermediates.
Trifluoromethanesulfonic acid esterification
Tf2O can convert oxygenated compounds into the corresponding trifluoromethanesulfonates, which are important intermediates in organic synthesis and are used for nucleophilic activation followed by conversion of amides, sulfoxides, and phosphorus oxides.
Trifluoromethyl sulphide reaction
Tf2O can be used as a trifluoromethylthio radical reagent. Hydrotrifluoromethylthiolation of unactivated alkenes and alkynes with Tf2O in the presence of PMePh2 and H2O under visible-light photoredox catalysis gave the addition products. The trifluoromethylthio radical (.SCF3) was first formed from Tf2O through a photoredox radical processes and deoxygenative reduction of PMePh2, and H2O serves as the H-atom donor for the hydrotrifluoromethylthiolation reaction. This reaction provides a new strategy for radical trifluoromethylthiolation.
Glycochemistry
Tf2O also has important uses in glycochemistry, such as Tf2O participation in sulfide or sulfoxide-induced dehydration Glycosylation reactions.

References:
[1] HAI HUANG J K. Triflic Anhydride (Tf2O)-Activated Transformations of Amides, Sulfoxides and Phosphorus Oxides via Nucleophilic Trapping[J]. Synthesis-Stuttgart, 2021, 25 1: 35-60. DOI:10.1055/a-1679-8205.[2] YAO OUYANG Prof.??Dr. F L Q Dr Xiu Hua Xu. Hydrotrifluoromethylthiolation of Unactivated Alkenes and Alkynes with Trifluoromethanesulfonic Anhydride through Deoxygenative Reduction and Photoredox Radical Processes[J]. Angewandte Chemie International Edition, 2019, 58 51: 18299-18714. DOI:10.1002/anie.201911323.
[3] ZAHRA TASHRIFI. Triflic Anhydride (Tf2O): An Efficient Catalyst for Electrophilic Activation of Amides[J]. ChemistrySelect, 2021, 6 21: 5166-5373. DOI:10.1002/slct.202100305.
Related articles And Qustion
See also
Lastest Price from Trifluoromethanesulfonic anhydride manufacturers

US $0.00-0.00/KG2025-05-15
- CAS:
- 358-23-6
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS

US $0.00/G/KG2025-04-22
- CAS:
- 358-23-6
- Min. Order:
- 1G/KG
- Purity:
- 99%
- Supply Ability:
- 100MT