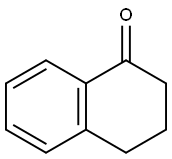
Variety Reactions Related to 1-Tetralone
1-Tetralone can be reduced in liquid ammonia to 1,2,3,4-tetrahydronaphthalene via a Birch reduction with lithium. The keto group can also be reduced to a secondary alcohol giving 1-tetralol, when a modified process is applied, using the addition of aqueous ammonium chloride solution after evaporation of the ammonia.

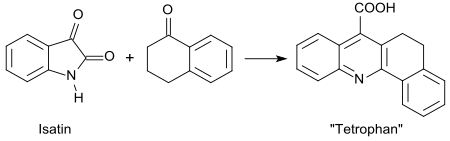
In the Pfitzinger reaction of 1-tetralone with isatin, a compound called tetrofan is formed.
The formed tertiary alcohol reacts with acetic anhydride upon elimination of water to 1-phenyl-3,4-dihydronaphthalene in the Grignard reaction of 1-tetralone with phenylmagnesium bromide, which is dehydrated with elemental sulfur in an overall yield of about 45% to 1-phenylnaphthalene.

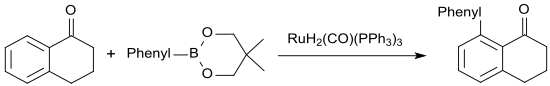
If use phenyl boronic acid neopentyl glycol ester, the ruthenium(II)-catalyzed arylation of 1-tetralone will give 8-phenyl-1-tetralone in up to 86% yield.
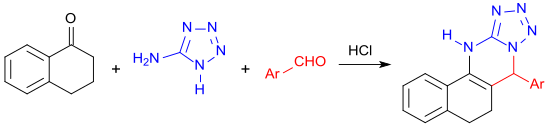
The four-membered heterocyclic ring system formed in a multi-component reaction under microwave irradiation of 1-tetralone with 5-aminotetrazole and an aromatic aldehyde.
You may like
Related articles And Qustion
Lastest Price from 1-Tetralone manufacturers

US $0.00/KG2025-04-21
- CAS:
- 529-34-0
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $290.00-150.00/kg2025-04-21
- CAS:
- 529-34-0
- Min. Order:
- 1kg
- Purity:
- 99% Purity (What/sapp: +86 18145728414)
- Supply Ability:
- 1000 Tons/Month