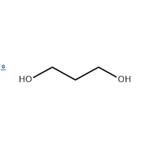
1,3-Propanediol Production
1,3-Propanediol, trimethylene glycol, HOCH2CH2CH2OH, is produced on a much smaller scale than its isomer, propylene glycol. In spite of some interesting areas of application, total production remains relatively small. The difficulties of manufacturing the product result in a poorly competitive price structure relative to other diols.

Chemical properties
1,3-Propanediol is a clear, colorless, odorless liquid that is miscible with water, alcohols, ethers, and formamide. It is sparingly soluble in benzene and chloroform. The chemical properties of 1,3-propanediol are typical of alcohols. Like 1,2-propanediol, 1,3-propanediol condenses with carboxylic acids at elevated temperature to yield esters. It also reacts with isocyanates and acid chlorides to yield urethanes and esters, respectively. Unlike 1,2-propanediol, 1,3-propanediol has two primary hydroxyl groups with equivalent reactivity.
Production
1,3-Propanediol is prepared by a two-step process involving the hydrolysis of acrolein to 3-hydroxypropanal followed by hydrogenation :
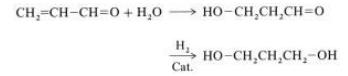
Hydrolysis is carried out under weakly acidic conditions in water containing initially ca. 20 % acrolein. Higher concentrations of acrolein tend to lead to greater amounts of undesired byproducts as a result of reaction between acrolein and hydroxypropanal.
3-Hydroxypropanal can be hydrogenated in the aqueous phase directly; however, the preferred technique is to extract the aldehyde into an organic solvent – particularly 2-methylpropanol – and then hydrogenate the aldehyde to yield the diol. Hydrogenation is conducted with Raney nickel under pressure in the aqueous phase and with nickel-supported catalysts at 2 – 4 MPa and 110 – 150 ◦C in the organic phase. The diol is subsequently separated from solvent and water by distillation.
The yield of desired product by this route is relatively low – approximately 45 %. Alternative techniques for synthesizing the diol have appeared in the patent literature. Hydroformylation of ethylene oxide followed by hydrogenation yields 1,3-propanediol in good yield (92 %), but a high catalyst concentration and a very large excess of solvent render the process uneconomical. More recently, hydroformylation of ethylene oxide directly to 1,3-propanediol with a rhodium – phosphine catalyst system has been disclosed. The reaction of ethylene with formaldehyde and carboxylic acids has also not been commericialized because of low selectivity.
You may like
Related articles And Qustion
Lastest Price from 1,3-Propanediol manufacturers
US $0.00-0.00/KG2025-12-03
- CAS:
- 504-63-2
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $0.00/kg2025-08-05
- CAS:
- 504-63-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 200 tons