Synthesis and Application of Chloramine B
General description
Chloramine B is an organochlorine disinfectant, mainly used for disinfection of drinking water utensils, various utensils, fruits and vegetables (5 ppm), aquaculture water and enamel utensils (1%). Can also be used for milk and milking cup cleaning and livestock urinary tract and purulent wound flushing disinfection.
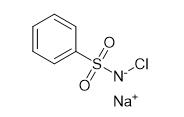
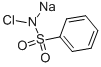
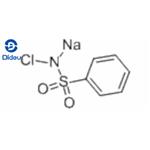
Fig. 1 The structure of Chloramine B.
Synthetic routes
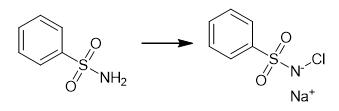
Fig. 2 The synthetic of Chloramine B.
It is prepared by the action of chlorine on benzenesulfonamide in the presence of NaOH. Benzenesulfonamide (1 mol) was added gradually to 4-5 N NaOH solution (2-3 mol) at 298K with stirring. When the solution became homogeneous, it was filtered, and the filtrate was heated to 338-343 K. Chlorine was bubbled slowly over a period of 1 h. The mass was stirred for 1 h at the same temperature, then heated to 358K and filtered through a Schott's funnel. Yield 99% [1].
Application
Bactericidal effect
The sporicidal effect of Presept was compared with Chloramine B on the spores of Bacillus cereus. Either compound was calibrated to the same concentration of active chlorine. While a portion of spore population after 4 hrs of treatment by Chloramine germinated and started to divide in a rich nutrient medium, the optical density of the culture inoculated with spores treated by Presept did not increase even after 7 hrs when exposed to the nutrient medium. Significant morphological differences were found in either population of spores. Spores treated by Presept lost the impermeability within 3 hrs in the nutrient medium but almost no postgerminative development was observed. However, a portion of spores treated by Chloramine B developed after germination within 3 hrs into vegetative cells. It seems that Presept does not block germination and/or loss of impermeability of spores, but prevents their postgerminative development and division [2].
The microbicidal effect of the disinfection remedies Dikonit, Chloramine B, Jodonal B and peracetic acid was followed in 50 strains of P. aeruginosa, 20 of them being in the growth phase M and 30 others in the phase R. The suspension method was used to determine effective concentrations and exposures. Differences in sensitivity were found among individual strains of P. aeruginosa to disinfection agents; small differences were found between the growth phases R and M only with Jodonal B at the 0.01% concentration. Although there were some exceptions, there was a general rule that a strain sensitive to one disinfection agent was also sensitive to the other and vice versa. In the experiments the most efficient remedy against the strains of P. aeruginosa was Dikonit followed by peracetic acid, Jodonal B, and Chloramine B [3].
Thermochemical study
The heat of reaction of Sodium Benzensulfonamide and Sodium Hypochlorite concentrated solutions, to obtain crystallized chloramine B, as well as the heat of reaction of the same substances in dilute solutions to obtain the chloramine B in solution, and the heat of solution of the crystalline chloramine B, have been measured calorimetrically, at 20-degrees-C. The obtained values, DELTAH(r)=-16,705 kcal/mole, DELTAH(r(soln))=-10,769 kcal/mole and DELTAH(dissln)satn=+5,941 kcal/mole, satisfy the relation showing that the first one is the sum of the two last ones, in the limits of the experimental uncertainty of +/-0.01 kcal/mole [4].
Kinetic Study
The oxidation of aliphatic primary amines, n-propylamine (PA) and [image omitted]-butylamine (BA), by chloramine-B (CAB) and chloramine-T (CAT) in aqueous alkaline medium has been kinetically studied at 318 K. The reaction follows the experimental rate law: rate [image omitted] k' [oxidant] [amine]0 [OH-]x, where oxidant is CAT or CAB and x is fractional. Additions of chloride ions, the reduction product, sulfonamide, and sodium perchlorate, which is used for changing the ionic strength of the solvent medium, have no effect on the reaction rate. However, the rate varies with change in dielectric constant of the medium. The reaction has been studied in the temperature range, 313-323K, and the activation parameters have been evaluated. A suitable mechanism consistent with the observed kinetic data has been proposed and the rate law derived [5].
References
[1] Jagadeesh R V, Sandhya Y S, Karthikeyan P, et al. The efficient palladium-catalyzed selective t synthesis of benzenesulfonic acids[J]. Synthetic Communications, 2011, 41(16): 2343-2349.
[2] Hlavácek O, Vinter V, Chaloupka J. Sporicidal effect of Presept and Chloramine B on Bacillus cereus spores[J]. Epidemiologie, Mikrobiologie, Imunologie: Casopis Spolecnosti pro Epidemiologii a Mikrobiologii Ceske Lekarske Spolecnosti JE Purkyne, 1998, 47(4): 150-153.
[3] Melichercíková V. Determination of microbicidal effect of selected disinfection remedies against various strains of Pseudomonas aeruginosa isolated from clinical material (author's transl)[J]. Ceskoslovenska Epidemiologie, Mikrobiologie, Imunologie, 1981, 30(2): 105-112.
[4] Sahini V E, Tomus E J. THERMOCHEMICAL STUDY OF THE REACTION BETWEEN SODIUM BENZENSULPHONAMIDE AND SODIUM-HYPOCHLORIDE, OBTAINING N-CHLORINATED SODIUM BENZENSULPHONAMIDE (CHLORAMINE-B)[J]. REVISTA DE CHIMIE, 1993, 44(1): 50-52.
[5] Veeraiah M K, Murthy A S A, Gowda N M M. Oxidation of n-Propylamine and n-Butylamine by Chloramine-B and Chloramine-T in Basic Medium: A Kinetic Study[J]. Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry, 2010, 40(4): 225-230.
You may like
See also
Lastest Price from Chloramine B manufacturers
US $0.00-0.00/kg2025-11-11
- CAS:
- 127-52-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $10.00/KG2025-04-21
- CAS:
- 127-52-6
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt