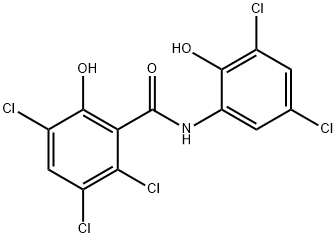
Oxyclozanide: Veterinary Applications, Antimicrobial Synergy, and Toxicity Profile
Oxyclozanide is a fasciolicide drug used to treat liver fluke and rumen fluke infections in ruminants and horses. It is also an antiparasitic active ingredient used in veterinary medicine against internal parasites (mainly flukes) of livestock. It is not used against agricultural and household pests. It belongs to the chemical class of the salicylanilides. It is ineffectice against roundworms, tapeworms or external parasites. It is used scarcely in ruminants (mainly in drenches) but not in other livestock, horses or pets. Oxyclozanide is available in the form of drenches, often mixed with a broad-spectrum nematicide.

The ionophore oxyclozanide enhances tobramycin
Scientists show that the ionophore anthelmintic oxyclozanide, combined with tobramycin, significantly increased killing of P. aeruginosa biofilms over each treatment alone. This combination also significantly accelerated the killing of cells within biofilms and stationary phase cultures and it was effective against 4/6 CF clinical isolates tested, including a tobramycin-resistant strain. The combination was effective against CF clinical isolates, including a tobramycin-resistant isolate and multiple S. aureus isolates growing as biofilms. We further show that oxyclozanide both permeabilized P. aeruginosa and functioned as a proton ionophore, reducing the membrane potential (Δψ) of P. aeruginosa. To confirm the results of the screen, P. aeruginosa PAO1 biofilms were exposed to 100 μM oxyclozanide or 500 μM tobramycin (∼500-fold the planktonic MIC), alone and in combination, for 6 h and the efficacy was determined using BacTiter-Glo. Oxyclozanide and tobramycin alone resulted in ∼2-fold fewer viable cells within biofilms compared with untreated controls. However, the combination of oxyclozanide and tobramycin was more effective, eradicating 87% (7.7-fold reduction) of the cells within biofilms. Oxyclozanide combined with the aminoglycosides gentamicin or streptomycin killed 96% (25-fold reduction) and 91% (11.1-fold reduction) of the cells within biofilms, respectively.[1]
Young CF patients are often colonized with Staphylococcus organisms, making tobramycin combined with oxyclozanide an applicable therapy for both early and late CF lung pathogens. Oxyclozanide has been reported to have two modes of action. It has been shown to function as an ionophore in parasitic worms and it was found to permeabilize the Gram-positive bacterium S. aureus growing planktonically. Our results indicate that it exhibits both activities against P. aeruginosa biofilms, which could contribute to the observed increase of cellular-associated tobramycin in the presence of oxyclozanide, and we suggest that each activity is important for it and tobramycin enhanced activity. Many bacterial species, including S. aureus and P. aeruginosa, can be found growing as biofilms in non-healing chronic wounds such as diabetic foot ulcers and burns. Previously, it has been shown that oxyclozanide has activity against planktonic S. aureus and cancerous cells, indicating broad applicability. The repurposing of veterinary drugs has a proven history of success, notably the ionophore anthelmintic ivermectin, which has been repurposed for the treatment of several diseases in humans. Oxyclozanide combined with tobramycin could be a potential new treatment for P. aeruginosa and S. aureus infections in CF patients as well as diabetic foot ulcers and burn wounds.
Toxicity Assessment of Oxyclozanide in Wistar Rats
Since no vaccine has been successfully developed, the effective strategy for controlling fascioliasis depends mainly on the use of pharmaceuticals (fasciolicide). Oxyclozanide was developed in 1966 and has achieved an effective effect in subsequent clinical applications. In clinical trials, oxyclozanide has been found to be effective not only for the treatment of F. hepatica infection but also for other tissue parasites, such as Hymenolepis sp. and Paramphistomum sp. According to Rana's recent review, oxyclozanide was effective on mature fluke, especially for adults over 14 weeks old. Additionally, a resistance of triclabendazole and albendazole in sheep, cattle, and water buffalos was reported widely. Fortunately, oxyclozanide resistance has still not been reported in animals and has a very good killing effect on F. hepatica resistant to triclabendazole. Maiden and co-workers defined it as an anti-biofilm agent that enhances the activity of aminoglycosides and tetracycline against Pseudomonas aeruginosa biofilm by reducing membrane potential, penetrating cells, and enhancing accumulation of tobramycin in biofilm. Those studies have attracted much more attention for the application of oxyclozanide.[2]
The biochemical analyses showed that AST was different between oxyclozanide-treated and untreated control groups. It is known that AST and ALT are released into the serum after relative cells and tissues were damaged. In most toxicological studies, the increase of ALT is generally considered to be a practical and specific enzyme indicator of hepatocyte injury. However, our histopathological studies showed no pathological changes in brains between the treatment and the controls. It suggests that oxyclozanide doesn't show damage to the nervous system under subacute exposure. Besides, the histology of duodenum showed that there was catarrhal enteritis in the normal architecture resulting from diarrhea after administration. The amount of serum AST may increase during hepatocyte injury. The LD50 of oxyclozanide was 3,707 mg/kg BW for experimental rats in both sex in the acute study. During the 28-day administration, differences in the histopathological, hematological, and biochemical assays were found at the 185- and 370-mg/kg dose groups vs. the untreated control group. These results suggest that oxyclozanide could cause damage in the liver, kidney, and heart. According to those results, the LOAEL level was 74 mg/kg for rats during the 28-day toxicity study, which provides basis for clinic use of oxyclozanide and for determining a reasonable safe dose.
Efficacy of Oxyclozanide against Rumen Flukes
The progressive increase of prevalence and reports of acute cases has led to the consideration of paramphistomosis as an emergent disease of ruminants in Europe. It has been suggested that this emergence may be the consequence, among others, of changes in climate conditions, importation of infected livestock, availability of more accurate diagnostic techniques, continuous deworming with anthelmintics ineffective against paramphistomids and the good adaptation of the parasite to Galba truncatula, the major intermediate host in Europe. The usefulness of closantel is controversial since high and limited efficacy has been reported in cattle. In contrast, most investigations reported oxyclozanide as highly efficient against both juvenile and adult paramphistomids in cattle and goats. Our results reveal that a single dose of oxyclozanide at 15 mg/kg is very efficient against paramphistomid infections in sheep since a significant reduction in egg shedding was observed between treated and control animals and effectiveness remained above 90% during the first 11 weeks after treatment.[3]
Our data suggest that oxyclozanide eliminated all adults and juvenile flukes in a small percentage of animals, since they began to shed eggs after the prepatency period. According to the present study, the situation of pharmacological control of paramphistomids in sheep is far from desirable. However, this study agrees with previous research reporting that oxyclozanide is an efficient and reliable anthelmintic for treating adult paramphistomids affecting domestic ruminants. In addition, our results show that G-OXI animals shed fewer eggs than G-CLS and G-CON, thus treating animals with oxyclozanide also reduces environment contamination with paramphistomid eggs and, consequently, infection in the intermediate host. In summary, Oxyclozanide is the only anthelmintic showing a good performance against rumen flukes currently available in sheep; thus, strategic treatments together with complementary control measures for reducing pasture parasitic burden are strongly recommended. Without other effective drugs available and rumen fluke prevalences increasing in Europe, finding new alternatives for the control of rumen flukes should be considered a priority.
References
[1]Maiden MM, Zachos MP, Waters CM. The ionophore oxyclozanide enhances tobramycin killing of Pseudomonas aeruginosa biofilms by permeabilizing cells and depolarizing the membrane potential. J Antimicrob Chemother. 2019 Apr 1;74(4):894-906. doi: 10.1093/jac/dky545. PMID: 30624737; PMCID: PMC6735725.
[2]Wang W, Dong Z, Zhang J, Zhou X, Wei X, Cheng F, Li B, Zhang J. Acute and Subacute Toxicity Assessment of Oxyclozanide in Wistar Rats. Front Vet Sci. 2019 Sep 6;6:294. doi: 10.3389/fvets.2019.00294. Erratum in: Front Vet Sci. 2023 Feb 01;10:1130466. doi: 10.3389/fvets.2023.1130466. PMID: 31552282; PMCID: PMC6743374.
[3]García-Dios D, Díaz P, Viña M, Remesar S, Prieto A, López-Lorenzo G, Cao JMD, Panadero R, Díez-Baños P, López CM. Efficacy of Oxyclozanide and Closantel against Rumen Flukes (Paramphistomidae) in Naturally Infected Sheep. Animals (Basel). 2020 Oct 22;10(11):1943. doi: 10.3390/ani10111943. PMID: 33105640; PMCID: PMC7690378.
You may like
Lastest Price from Oxyclozanide manufacturers

US $0.00/KG2025-04-21
- CAS:
- 2277-92-1
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 10tons/month

US $4.00/kg2025-04-21
- CAS:
- 2277-92-1
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000