Preparation and Application Phosphorous acid
General description
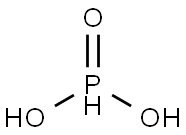
Phosphorous acid is an inorganic compound whose chemical formula is H3PO3 and molecular weight is 82.00. Soluble in water and alcohol. It is slowly oxidized into phosphoric acid in air and decomposed into phosphoric acid and phosphine (highly toxic and explosive) when heated to 180℃. Phosphite is a diacid, which is slightly more acidic than phosphoric acid. It has strong reducibility, easy to reduce silver ion (Ag+) to silver metal (Ag), and can reduce sulfuric acid to sulfur dioxide. Strong hygroscopicity and deliquescence, corrosive. Phosphite is mainly used as reducing agent, nylon whitening agent, also used as phosphite raw material, pesticide intermediates and organic phosphorus water treatment agent raw material.
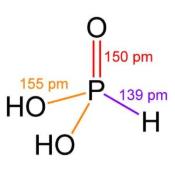
Fig. 1 The structure of Phosphorous acid.
Preparation
1. While stirring, the phosphorus trichloride was slowly added to the water to control the acceleration of the drops, so that the reaction would not be too intense, and the reactor was cooled with ice water. At the end of the reaction, the filtrate was filtered, heated below 140℃ and evaporated to one third of the original volume to drive away the hydrogen chloride gas, and then cooled to 60-85℃. A small amount of phosphite crystal was added as the crystal species, and the phosphite was further cooled and crystallized under stirring.
2. Take a 1000 mL three-neck round-bottom flask and assemble the machine stirrer, drip funnel and condenser (the outlet is connected to the HCl absorber). Put distilled water in a flask and cool it. Under stirring, 100 mL of newly distilled PCl3 (boiling range 74 ~ 76℃) was slowly added from a drip funnel. The filtrate was filtered and evaporated to 1/3 of its original volume at less than 140 ° C. It was cooled to 60 ~ 65℃, and H3PO3 grains were sprinkled as seed, cooled and crystallized under stirring. The output is about 63g. For further purification, 10 g of the product can be dissolved in 3.5 g water first, and then the rest of the whole part is added, carefully stirred, filtration crystallization, the yield of 40 g. Place in a ground glass bottle.
3. In the same device as above, 300 mL CCl4 and 100 g newly steamed PCl3 were put into the flask, chilled, stirred, and slowly added 75 g distilled water from the drip funnel. After adding all the water, remove the water bath and accelerate stirring for 1 h. Transfer the reactants to a separation funnel and wash with four times the amount of CCl4 (add 2 ~ 3 mL water if crystallization precipitates). The washed acid solution was filtered through a glass funnel and transferred to a flask. Under reduced pressure, the solution was evaporated over a water bath (not more than 60 ° C) and HCl was removed for 3 h. It is then placed in a desiccator to cool and crystallize.
Application
The effects on the oxygen reduction reaction
The effects of phosphorous acid additions on the oxygen reduction reaction at platinum electrodes in concentrated phosphoric acid were studied. The oxygen reduction currents decreased, and the Tafel slopes became more negative upon the addition of small concentrations of phosphorous acid. In addition, the phosphorous acid oxidation current tended to compete with the oxygen reduction current. These effects became more pronounced at higher phosphorous acid concentrations and at higher temperatures. Upon the addition of phosphorous acid the number of electrons involved in the oxygen reduction reaction decreased from a value close to four to a value approaching two, suggesting promotion of a two-electron reduction to peroxide. Therefore, in studies of the electrochemical reduction of oxygen in hot concentrated phosphoric acid or in fuel cell systems using hot concentrated phosphoric acid as electrolyte, it is recommended that precautions be taken against the inadvertent formation of phosphorous acid. The removal of phosphorous acid from concentrated phosphoric acid by repeated potential cycling at 100 mV/s between +0.5 and +1.50 V (vs. dynamic hydrogen electrode) was demonstrated [1].
Modified TiO2 nanoparticle photocatalysts
TiO2 nanoparticles synthesized by a sol-hydrothermal process were modified with phosphorous acid by an impregnation method. The effects of phosphorous acid modification on the thermal stability and photocatalytic activity of anatase TiO2 were mainly investigated. The results show that the phosphorous acid modification obviously enhances the thermal stability of nanosized anatase TiO2, a principal anatase phase was maintained even after thermal treatment at 800 degrees C. During the processes of photocatalytic degradation RhB, the as-prepared phosphorous acid modified TiO2 by thermal treatment at 700 degrees C exhibits higher activity than P25-TiO2 [2].
Control Phytophthora diseases
Phosphorous acid is known to effectively control various Oomycetes diseases. The phosphoric acid moves upward and downward through the xylem and phloem in plants. The sustainable forms of the slow releasing chemical in rhizosphere would be ideal to be up-taken by plants. Therefore, Park et al. developed a new system for phosphorous acid formulation using a carrier coated with polysaccharides. When the product was applied in rhizosphere, the adequate amount of phosphorous acid was consistently released up to 4 weeks in rhizosphere soils. While soil drenching with phosphorous acid at 1,000 mu g/ml and metalaxyl at 150 mu g/ml were not effective to control pepper Phytophthora blight for 4 weeks, direct application of our formulation product around basal stem of pepper plants resulted in excellent disease control effect against Phytophthora blight over 4 weeks. The application of 4 g of our product per plant was optimum to control the disease, and 8 g product/plant did not cause phytotoxicity. Based on the results, we conclude that the applications of the formulation product once or twice during cropping season can control Phytophthora diseases on various crops [3].
References
[1] Sugishima N, Hinatsu J T, Foulkes F R. Phosphorous acid impurities in phosphoric acid fuel cell electrolytes: II. Effects on the oxygen reduction reaction at platinum electrodes[J]. Journal of the Electrochemical Society, 1994, 141(12): 3332.
[2] Xu Q, Li-Qiang J, Lian-Peng X, et al. Phosphorous acid modified TiO2 nanoparticle photocatalysts[J]. Chinese Journal of Inorganic Chemistry, 2008, 24(7): 1108-1112.
[3] Park H J, Kim S H, Jee H J. A new formulation system for slow releasing of phosphorous acid in soil for controlling Phytophthora diseases[J]. The Plant Pathology Journal, 2007, 23(1): 26-30.
You may like
See also
Lastest Price from Phosphorous acid manufacturers
US $10.00/kg2025-04-21
- CAS:
- 13598-36-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100

US $50.00-1200.00/kg2025-04-17
- CAS:
- 13598-36-2
- Min. Order:
- 1000kg
- Purity:
- 0.99
- Supply Ability:
- 50000 Metric Ton / Metric Tons per Year